All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Do you know... T-cell exhaustion is a mechanism of resistance to BCMA-directed therapies. It is observed least frequently with which type of BCMA-directed therapy?
Video series
At the ESH 7th Translational Research Conference on Multiple Myeloma in 2024, the Multiple Myeloma Hub held a symposium titled: How to sequence B-cell maturation antigen (BCMA)-directed therapies in early relapsed/refractory multiple myeloma (RRMM). During the symposium, Martin Kaiser, The Royal Marsden Hospital, London, UK, delivered a presentation on BCMA-directed antibody–drug conjugates (ADCs) for the treatment of MM.
In this presentation, Professor Kaiser focuses on the potential and evolving role of ADCs in the treatment of multiple myeloma, particularly in comparison to other BCMA-targeting therapies such as bispecific antibodies and chimeric antigen receptor (CAR) T-cell therapies. The discussion includes mechanisms of action (Figure 1), recent clinical trial data, toxicity profiles, resistance mechanisms (Figure 2), and future directions for optimizing ADC use. The efficacy of belantamab mafodotin in triplet regimens and its potential role in therapy sequencing are also covered.
Figure 1. BCMA-directed ADCs: Mechanism of action*
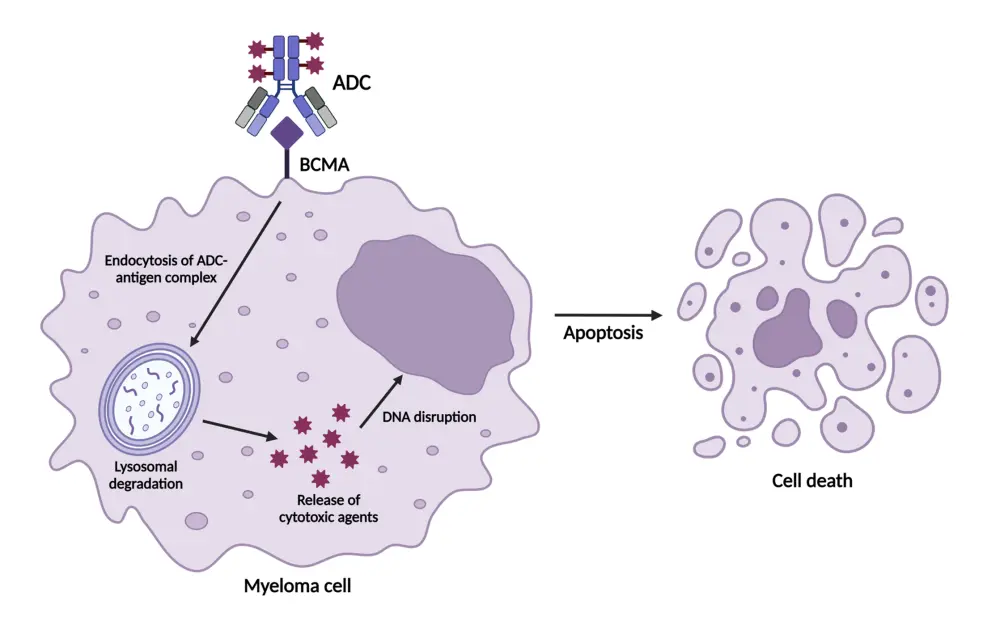
ADC, antibody–drug conjugate; BCMA, B-cell maturation antigen; DNA, deoxyribonucleic acid.
*Khattak et al.1
Figure 2. Impact of ADCs on mechanisms of resistance: T-cell independence*
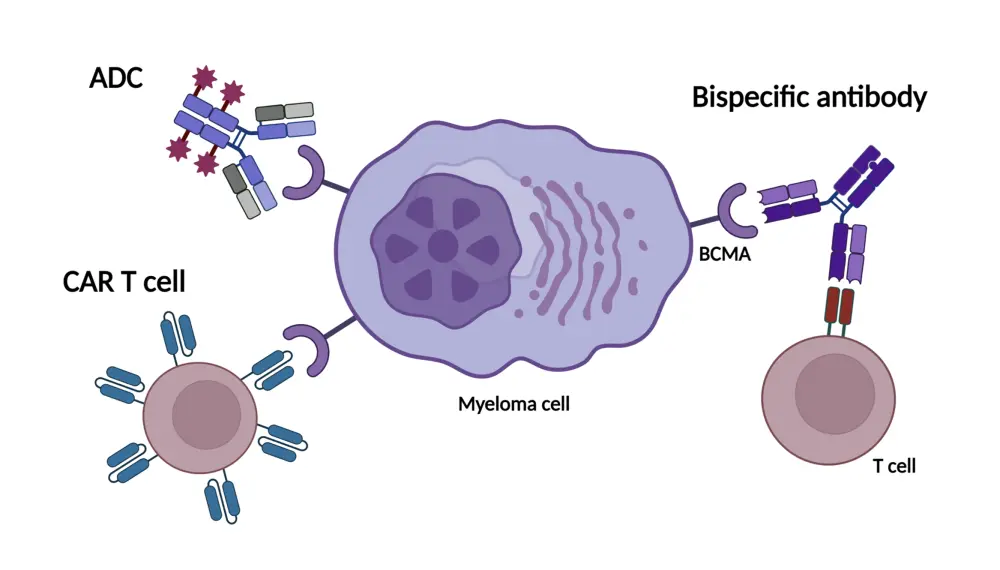
ADC, antibody–drug conjugate; BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor.
*Su and Ye.2
Key points
Mechanism of action
- ADCs selectively target cells by binding to specific antigens, such as BCMA, expressed on the surface of myeloma cells; this specificity minimizes damage to surrounding tissues.
- ADCs deliver a cytotoxic payload directly to myeloma cells without engaging T cells.
- Several ADCs are currently under investigation for the treatment of MM; among these, belantamab mafodotin is the most advanced BCMA-targeted ADC, with data available from phase III clinical trials.
DREAMM-7
- The phase III DREAMM-7 (NCT04246047) clinical trial evaluated belantamab mafodotin in combination with bortezomib and dexamethasone (BVd) compared with standard daratumumab, bortezomib, and dexamethasone (DVd) therapy.
- The overall response rate increased numerically from 71% in the DVd group to 83% in the BVd group.
- The complete response measurable residual disease negativity rate significantly improved, rising from 9.6% in the DVd group to 24.7% in the BVd group.
- Median progression-free survival was substantially longer with the BVd regimen, at 36.6 months compared with 13.4 months with the DVd regimen.
- The efficacy rates observed with the BVd regimen were maintained even after bortezomib discontinuation, demonstrating promising durability.
DREAMM-8
- The phase III DREAMM-8 (NCT04484623) clinical trial evaluated belantamab mafodotin in combination with pomalidomide and dexamethasone (BPd) vs daratumumab, pomalidomide, and dexamethasone (DPd).
- Overall response rate was higher in the BPd group at 77% compared with 72% in the PVd group.
- Complete response measurable residual disease negativity rate was significantly increased with BPd, at 24% compared with 5% in the PVd arm.
- At the time of analysis, median progression-free survival was not reached in the BPd group, compared with 12.7 months in the PVd group, indicating sustained benefits with BPd therapy.
- These results align closely with those from DREAMM-7, reinforcing the potential of belantamab mafodotin combination therapies in MM.
Ocular toxicities
- The most common ocular events noted with belantamab mafodotin included corneal changes and mild visual impairments, such as dry eyes and visual disturbances.
- Severe visual impairment, defined as bilateral worsening of best-corrected visual acuity to 20/200 or worse, was rare, occurring in 2% of DREAMM-7 patients and 1% of DREAMM-8 patients who received belantamab mafodotin.
- As the corneal epithelium regenerates regularly, recovery can occur after treatment pauses or dose adjustments of belantamab mafodotin.
- Most patients, including those with higher-grade toxicity, experienced full recovery or substantial improvement following dose adjustment.
- Although ocular toxicity requires monitoring and management, it does not significantly impact patients’ quality of life or long-term treatment outcomes.
Dosing strategies
- Emerging data supports less frequent dosing schedules to optimize efficacy and minimize toxicity.
- Lower doses or reduced dosing frequencies helped to manage toxicities, without sacrificing the efficacy of belantamab mafodotin.
- Extended dosing regimens could enhance the tolerability and accessibility of ADC therapies.
Resistance mechanisms and sequencing
- T-cell engagers require functional T cells, which can lead to T-cell exhaustion and limit their effectiveness.
- Unlike CAR T-cell therapies and bispecific antibodies, ADCs do not rely on T-cell activity.
- ADCs demonstrate sustained efficacy when used after other BCMA-targeted therapies, such as CAR T-cell therapies or bispecific antibodies.
- Additional studies are needed to establish optimal sequencing of ADCs with other therapies and explore the benefits of treatment-free intervals.
Clinical implications and future directions
- Belantamab mafodotin has shown efficacy and manageable toxicity, supporting its use in the early relapse setting and offering a promising option for patients in early relapse, particularly in cases where CAR T-cell or bispecific therapies are unavailable.
Your opinion matters
I will change my clinical practice as a result of this symposium
This independent educational activity was supported by GSK. All content was developed independently by the faculty. The funder was allowed no influence on the content of this activity.
References


 Martin F. Kaiser
Martin F. Kaiser




