All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Symposium | Panel discussion | Strategies for the management of treatment-related toxicities in MM
Do you know... Which of the following is NOT a method recommended to control the ocular side effects observed with belantamab mafodotin?
Video series
At the European School of Haematology (ESH) 7th Translational Research Conference on Multiple Myeloma in 2024, the Multiple Myeloma Hub held a symposium titled: How to sequence B-cell maturation antigen (BCMA)-directed therapies in early relapsed/refractory multiple myeloma (RRMM). Mohamad Mohty chaired a second panel discussion, joined by expert speakers Martin Kaiser and Rakesh Popat, focusing on strategies for the management of treatment-related toxicities in MM.
In this panel discussion, the speakers addressed key considerations in managing treatment-related toxicities, particularly with advanced and targeted therapies in MM. The conversation focused on the use of belantamab mafodotin in both relapsed and first-line settings, emphasizing the importance of optimizing quality of life and managing side effects, without sacrificing outcomes. The speakers explored belantamab mafodotin-based combinations, highlighting dosing strategies to minimize ocular toxicities (Figure 1).
Figure 1. Case discussion
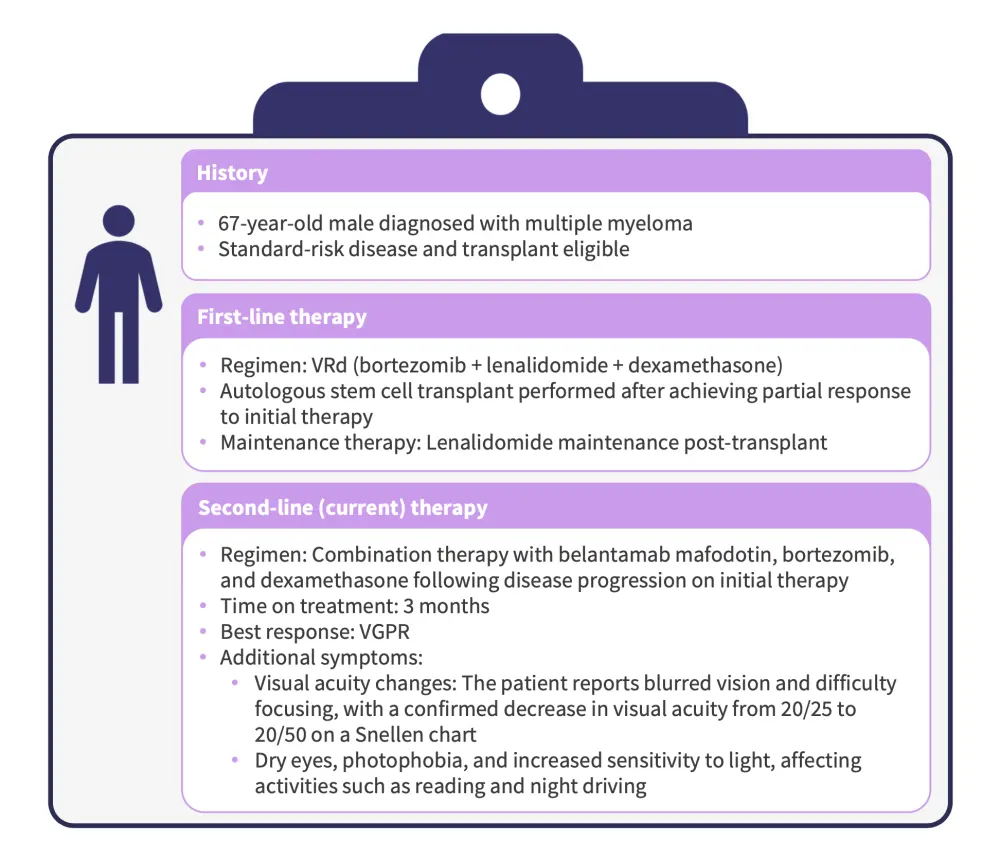
VGPR, very good partial response.
Key points
Treatment of early relapse in MM
- Early relapse is a crucial point in the treatment of MM, as it impacts the available options for subsequent therapies and affects patient quality of life.
- Patients who respond well to initial therapies, such as quadruplet therapy, are often fit at the time of relapse, allowing for the selection of less aggressive therapies that preserve quality of life; however, options are more limited for those who do not respond well to first-line treatment or for frail patients.
Belantamab mafodotin
- Belantamab mafodotin is a BCMA-directed antibody–drug conjugate currently being investigated for use in both the first-line setting and in early relapse of MM.
- There are several belantamab mafodotin -based combinations being explored, which demonstrate prolonged progression-free survival in clinical trials up to 36 months.
Ocular toxicity management
- Belantamab mafodotin is associated with ocular toxicities such as dry eyes, photophobia, and changes in visual acuity; these effects are typically related to corneal toxicity, which has been observed to resolve with time in most cases.
- Good hygiene and lubricating eye drops are essential for managing these symptoms, and the frequency of belantamab mafodotin dosing can also be adjusted to allow for eye recovery and mitigate toxicity.
- Baseline ocular health assessments are vital, as pre-existing conditions such as dry eyes and keratopathy can exacerbate the risk of ocular toxicity.
Adjustment of dosing regimens
- The introduction of induction and maintenance phases with belantamab mafodotin may be beneficial in reducing toxicities, with belantamab mafodotin typically starting at higher doses and transitioning to lower doses for long-term maintenance.
- In clinical practice, belantamab mafodotin is often administered at an initial dose of 2.5 mg/kg, stepping down to 1.9 mg/kg after achieving disease control.
- After 6 months, patients may transition to a maintenance strategy of belantamab mafodotin every 12 weeks.
- These adjustments allow time for the cornea to regenerate and help overcome toxicities, with the additional benefit of reducing hospital visits and being less disruptive for patients.
Selecting a treatment regimen
- The decision between different belantamab mafodotin combinations and other regimens, such as pomalidomide combinations, depends on patient-specific factors such as previous drug exposure and tolerability.
- Polyneuropathy and cytopenias influence the choice, with pomalidomide-based combinations being more suitable for patients with pre-existing neuropathy, while bortezomib mafodotin-based regimens may be better for those with a higher risk of cytopenias.
- Emerging data suggest that belantamab mafodotin may be effective first-line as well as in early relapse.
- Ongoing studies are also exploring the use of belantamab mafodotin in combination with other agents, such as carfilzomib, for enhanced efficacy in the first-line setting.
Your opinion matters
I will change my clinical practice as a result of this symposium
This independent educational activity was supported by GSK. All content was developed independently by the faculty. The funder was allowed no influence on the content of this activity.
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Mohamad Mohty
Mohamad Mohty Rakesh Popat
Rakesh Popat Martin F. Kaiser
Martin F. Kaiser


