All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Do you know... What percentage of patients are unable to receive treatment beyond the third-line of therapy for multiple myeloma?
Video series
At the ESH 7th Translational Research Conference on Multiple Myeloma in 2024, the Multiple Myeloma Hub held a symposium titled: How to sequence B-cell maturation antigen (BCMA)-directed therapies in early relapsed/refractory multiple myeloma (RRMM). During the symposium, Rakesh Popat, UCL Hospitals NHS Foundation Trust, London, UK, delivered an expert presentation on the existing and developing treatment paradigm for early RRMM.
In this presentation, Popat discusses the evolving treatment paradigm for early RRMM, highlighting emerging therapeutic approaches, the expansion of existing therapies into earlier treatment lines, and the latest clinical trial data. Popat emphasizes the importance of tailoring treatments based on patient-specific factors, such as genetic risk, transplant eligibility, and frailty (Figure 1), while considering the place for immunotherapies with different targets (Figure 2) in the early relapse setting. Popat discusses promising data associated with the use of BCMA-targeted therapies in earlier lines, as well as the challenges of sequencing these therapies across different patient populations and access issues globally.
Figure 1. Factors involved in choosing treatment regimens for RRMM*
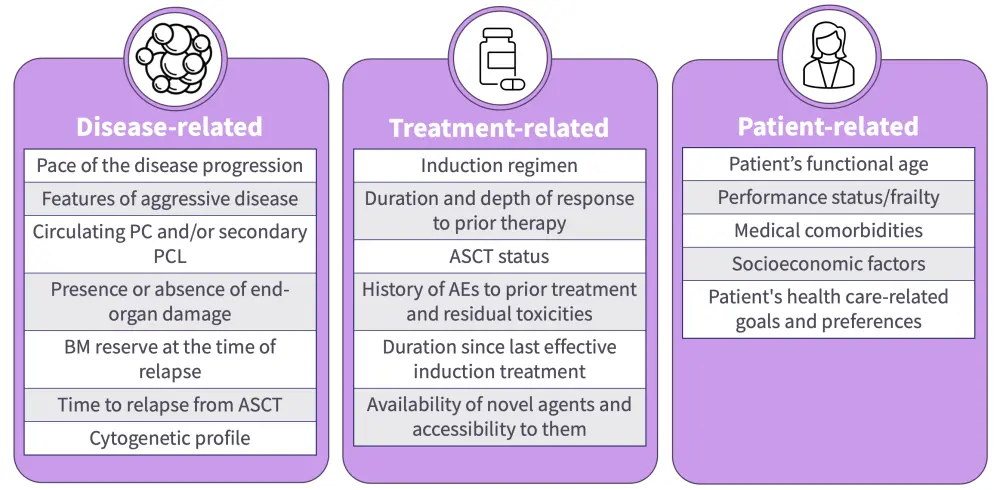
AE, adverse event; ASCT, autologous stem cell transplant; BM, bone marrow; PC, plasma cells; PCL, plasma cell leukemia; RRMM, relapsed/refractory multiple myeloma.
*Devarakonda et al.1
Figure 2. Targets for immunotherapies in multiple myeloma*
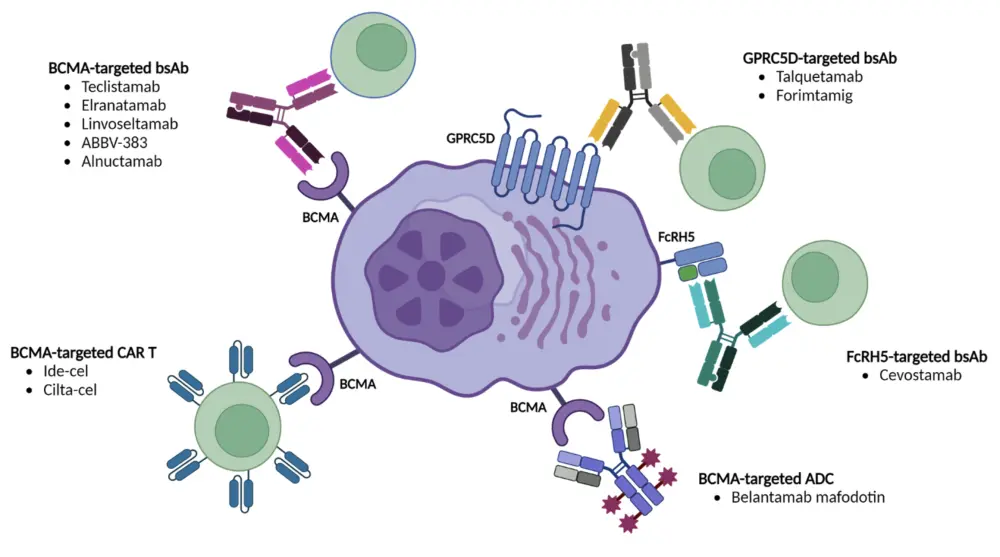
ADC, antibody–drug conjugate; BCMA, B-cell maturation antigen; bsAb, bispecific antibody; cilta-cel, ciltacabtagene autoleucel; FcRH5, Fc receptor homolog 5; GPRC5D, G protein-coupled receptor class C group 5 member D; ide-cel, idecabtagene vicleucel.
*Parrondo et al.2
Key points
- The MM treatment landscape is rapidly evolving, making current guidelines already outdated.
- Transplant eligibility is the key factor in determining treatment pathways in the newly diagnosed setting, patients can then be stratified based on genetic risk.
- For older, transplant-ineligible patients, both genetic risk and frailty must be considered, as frailty status may outweigh genetic factors in determining outcomes.
- A triplet regimen of daratumumab, lenalidomide, and dexamethasone remains the cornerstone of treatment for transplant-ineligible patients; however, frail patients may benefit more from a doublet regimen and earlier treatment cessation if necessary due to tolerability.
- Fitter patients typically experience higher response rates with quadruplet regimens such as daratumumab-bortezomib-lenalidomide-dexamethasone and isatuximab-bortezomib-lenalidomide-dexamethasone, particularly those with high-risk disease.
- Upon relapse, treatment options depend on the patient's response to lenalidomide and CD38 antibody-refractoriness.
- For patients refractory to both CD38 antibodies and lenalidomide, treatment becomes more complex, often involving newer agents like selinexor or carfilzomib, which remain unlicensed in some areas.
- A growing number of patients are becoming resistant to standard therapies, highlighting the need for more novel therapeutic options.
- The development of immune therapies has primarily focused on BCMA, but also include newer targets such GPRC5D and FcRH5.
- Several immune-based treatments, including chimeric antigen receptor (CAR) T-cell therapies and bispecific antibodies, have been approved based on positive outcomes in patients with heavily pretreated MM. However, there have been recent expansion of these therapies into earlier relapsed setting.
- Introducing these targeted therapies in earlier lines raises the challenge of sequencing, particularly when multiple BCMA-targeted options are available.
- Administering targeted therapies earlier may improve efficacy due to less pre-existing resistance, fitter T cells, and increased immunogenicity of tumor cells.
- Attrition rates decrease through later lines of therapy, with 85% being unable to receive treatment beyond the third-line of therapy, suggesting a need to move effective therapies to earlier lines where more patients may be eligible. However, using the most effective treatments early may complicate the management of subsequent lines, as certain options may no longer be viable in later stages.
- Access issues also vary globally, affecting the availability of CAR T-cell therapies or bispecific antibodies at different stages of relapse.
- Overall, effective treatment requires a balance of disease-related, treatment-related, and patient-related factors. Most importantly, for all patients it is vital to determine an individualized treatment plan.
Your opinion matters
I will change my clinical practice as a result of this symposium
This independent educational activity is supported by GSK. All content was developed independently by the faculty. The funder was allowed no influence on the content of this activity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Rakesh Popat
Rakesh Popat


