All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Symposium | Panel discussion | How to sequence BCMA-directed therapies in RRMM
Do you know... T-cell exhaustion is a mechanism of resistance to BCMA-directed therapies. It is observed least frequently with which type of BCMA-directed therapy?
Video series
At the European School of Haematology (ESH) 7th Translational Research Conference on Multiple Myeloma in 2024, the Multiple Myeloma Hub held a symposium titled: How to sequence B-cell maturation antigen (BCMA)-directed therapies in early relapsed/refractory multiple myeloma (RRMM). Mohamad Mohty chaired a panel discussion, focusing on strategies for sequencing BCMA-directed therapies in RRMM, joined by expert speakers Martin Kaiser and Rakesh Popat.
In this panel discussion, Mohty shared a case study of a 74-year-old female patient with RRMM, who experienced progression after 5 years of remission following treatment with isatuximab, bortezomib, lenalidomide, and dexamethasone (Isa-VRd) (Figure 1). This case prompted a discussion on the most effective and practical next-line treatments, considering the benefits and risks of options such as chimeric antigen receptor (CAR) T-cell therapy, bispecific antibodies, antibody–drug conjugates, and other novel combinations.
The panelists discussed treatment options for patients who have become refractory to existing therapies, the optimal sequencing of BCMA-directed therapies, and toxicity profiles of each option. They also highlighted the challenges of treatment accessibility, particularly in lower-income countries, as well as the importance of aligning with patient preferences.
Figure 1. Case study*
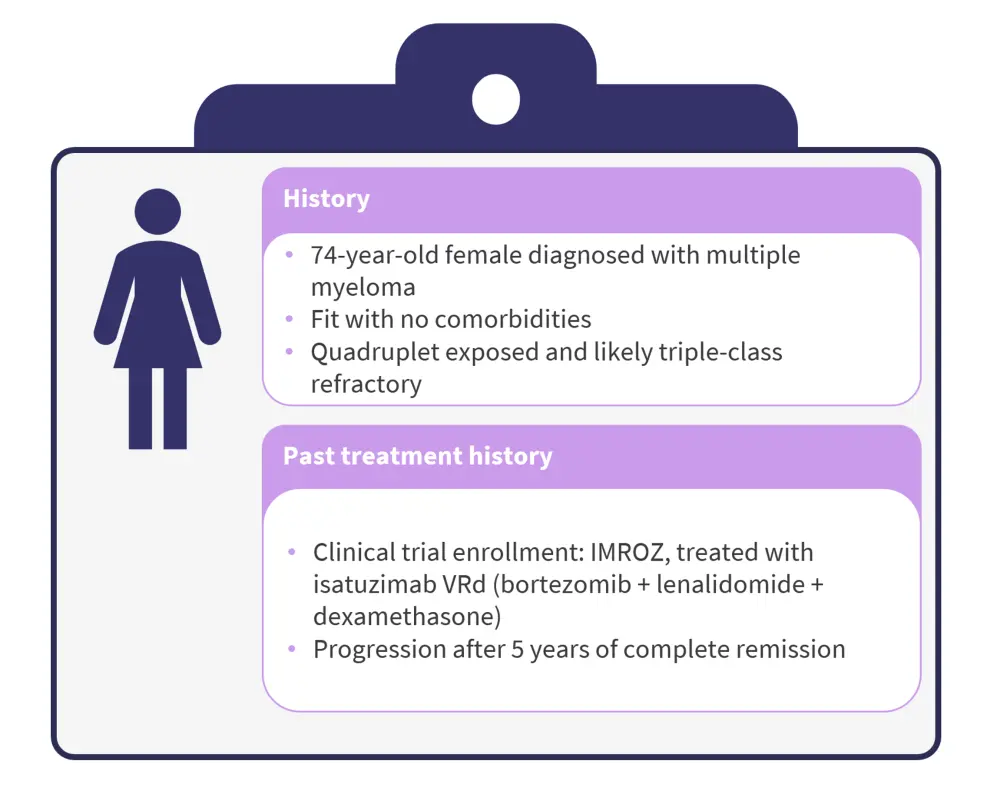
*Case provided by Mohty M.
Key points
- In patients who are triple-class exposed and experiencing disease progression, BCMA-targeted therapies may offer benefits, particularly for those who are fit and have few comorbidities.
CAR T-cell therapies
- BCMA-directed CAR T-cell therapies are an appropriate treatment choice for fit patients with no comorbidities; they have the benefit of being administered as a single infusion, and can be very effective in heavily pretreated MM.
- However, they are associated with significant neurotoxicity, increased risk of secondary malignancies, and require inpatient monitoring, making them unsuitable for patients who are older, frail, or unable or unwilling to receive in-hospital treatment.
- CAR T-cell therapies are approved as second- and fourth-line treatments in some regions, but optimal timing remains unclear.
- Delivering effective bridging therapy before CAR T-cell infusion may reduce the need for extensive T-cell expansion and potentially lower neurotoxicity risks.
Antibody–drug conjugates
- Belantamab mafodotin has shown efficacy in clinical trials, as well as benefitting from community administration and less frequent dosing, making it potentially more suitable for patients seeking less invasive or disruptive therapy.
- Latest data from studies such as the DREAMM-7 (NCT04246047) and DREAMM-8 (NCT04484623) trials suggest that combining belantamab mafodotin with other agents, for instance bortezomib or pomalidomide, can be effective, though prior treatment regimens and access need to be considered.
Bispecific antibodies
- Teclistamab and elranatamab have demonstrated efficacy and comparable safety profiles in trials; however, they may not be available as second-line therapies in some regions, which could limit their applicability. The choice between bispecific antibodies may also depend on factors such as weight-based versus fixed dosing and institutional preferences.
- Bispecific antibodies are available "off-the-shelf" and require fewer hospital visits and less intensive monitoring, making them suitable for older patients or those preferring less invasive treatment.
Carfilzomib-based regimens
- In cases where BCMA-targeted therapy is not available, carfilzomib-based regimens, such as carfilzomib, cyclophosphamide, and dexamethasone (KCd) or carfilzomib, pomalidomide, and dexamethasone (KPd), could be considered.
- Despite prior refractoriness to bortezomib, some patients may still respond to proteasome inhibitors, making carfilzomib a viable option; the weekly dosing schedule for carfilzomib can also benefit patients who prefer less disruptive treatment, although access and licensing issues may limit this option.
Additional considerations
- Minimizing treatment-related toxicity is essential, especially in older and frail patients; BCMA-directed therapies such as CAR T-cell and bispecific antibody therapies can result in mechanisms of resistance including T-cell exhaustion, whereas the use of antibody–drug conjugates minimize this and offer a less frequent dosing regimen.
- Patient preferences are also vital to consider and, for example, willingness to undergo intensive hospital stay versus a lower-intensity outpatient regimen may influence the treatment decision.
- Accessibility to novel and targeted therapies vary by region; for example, BCMA-targeted bispecific antibodies and CAR T-cell therapies are not yet approved for early-line use in some countries.
- Given that attrition rates decrease with each line of therapy, prioritizing the highest efficacy therapies in early relapse may help extend progression-free survival.
- Management of T-cell expansion is also important and the early use of tocilizumab and steroids can help mitigate the risks and reduce neurotoxicity.
Conclusions
- The primary goal of treatment is to optimize sequencing, minimize side effects, and maximize efficacy to improve patient outcomes.
- While there is no definitive consensus on the optimal sequence for BCMA therapies, using CAR T-cells or antibody–drug conjugates as initial options followed by bispecific antibodies may improve outcomes in later lines with other BCMA-directed therapies.
- CAR T-cells and bispecific antibodies are effective for the treatment of MM; however, their use should be individualized based on the patient characteristics, toxicity, treatment accessibility, and patient preferences.
- Testing for BCMA loss may guide therapeutic choices and enhance treatment efficacy.
- As new therapies emerge, ongoing research and clinical insights are essential to balance risks and benefits, ensuring optimal treatment selection for each patient.
Your opinion matters
I will change my clinical practice as a result of this symposium
This independent educational activity is supported by GSK. All content is developed independently by the faculty. The funder is allowed no influence on the content of this activity.
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Mohamad Mohty
Mohamad Mohty Rakesh Popat
Rakesh Popat Martin F. Kaiser
Martin F. Kaiser


