All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
A comprehensive guide on when and how to treat early relapsed multiple myeloma
In recent years, the treatment options for relapsed multiple myeloma (MM) have dramatically improved due to the introduction of novel agents and combinations with encouraging responses, even in patients with advanced disease. However, the availability of novel regimens brings new challenges such as managing toxicities, treatment personalization, sequencing treatments at relapse, patient treatment history, and personal preference.
At the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Amrita Krishnan presented an overview of the treatment landscape for both early and late relapsed MM and discussed the role of emerging immune-based therapies.1 Below is a comprehensive review that encompasses the results of the latest studies and some of Krishnan’s considerations when deciding the treatment course in the relapsed setting.
Treatment of early relapses
For the purposes of the study, early relapse is defined as first or second relapse. Patients who relapse after one or two treatment regimens still have many alternative therapies compared with patients with more advanced MM. When evaluating treatment options for relapsed MM (Figure 1), there are at least five key considerations to take into account2:
- The aggressiveness of the relapse – Patients who experience a biochemical relapse (asymptomatic) may not require an aggressive treatment plan and could be treated by increasing the dose or adding a synergetic drug. For example, if a patient experiences a biochemical relapse while receiving maintenance therapy with lenalidomide, this could be managed by increasing the lenalidomide dose or adding dexamethasone or a monoclonal antibody (mAb). Patients who relapse with aggressive MM, including renal failure or extramedullary disease, may be considered for a combination regimen with novel agents.
- Previous therapies – The most frequently used drug classes to treat MM are proteasome inhibitors (PIs), immunomodulatory (IMiD) agents, and mAb (CD38-directed mAbs, daratumumab and isatuximab, and anti-SLAMF7 mAb, elotuzumab). The choice of regimen is guided by response and tolerability to previous therapies. It is currently unclear whether a drug class switch is clinically superior to using drugs within the same class at the time of relapse.
- Previous autologous hematopoietic stem cell transplantation (auto-HSCT) – auto-HSCT is the primary treatment offered to patients who defer transplantation as initial treatment. Patients who do not respond to standard-dose treatment should remain eligible for salvage auto-HSCT.
- Comorbidities – In general, triplet regimens are preferred compared with doublets, except for extremely frail patients. Frail patients can be considered for PI-containing doublets, dexamethasone, IMiD-based therapies such as lenalidomide + dexamethasone and pomalidomide + dexamethasone, and daratumumab monotherapy.
- Access to care and personal preference – Finances, route of administration, and drug access are also important considerations when selecting a treatment plan. Patients who cannot travel may require an oral regimen. Those with financial challenges may not want oral medications and prefer an intravenous/subcutaneous medication.
Figure 1. Treatment options based on relapse characteristics
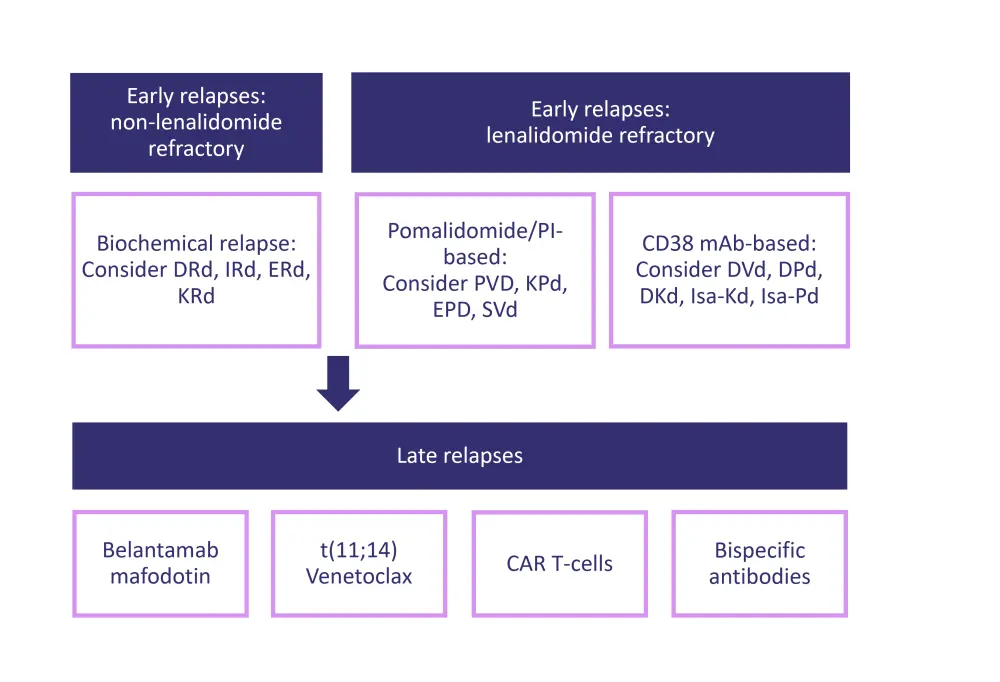
DKd, daratumumab + carfilzomib + dexamethasone; DPd, daratumumab + pomalidomide + dexamethasone; DRd, daratumumab + lenalidomide + dexamethasone; DVd, daratumumab + bortezomib + dexamethasone; EPD, elotuzumab + pomalidomide + dexamethasone; ERd, elotuzumab + lenalidomide + dexamethasone; IRd, ixazomib + lenalidomide + dexamethasone; Isa-Kd, isatuximab + carfilzomib + dexamethasone; Isa-Pd, isatuximab + pomalidomide + dexamethasone; KPd, carfilzomib + pomalidomide + dexamethasone; KRd, carfilzomib + lenalidomide + dexamethasone; mAb, monoclonal antibody; PI, proteasome inhibitor; PVD, pomalidomide + bortezomib + dexamethasone; SVd, selinexor + bortezomib + dexamethasone.
*Adapted from Nathwani et al.2
As the MM treatment paradigm evolves and quadruplet regimens are introduced, deciding which treatment is best for each patient in relapse becomes more difficult. Currently, most patients in the first relapse are initially treated with a PI and IMiDs, and are anti-CD38-naïve, hence they are eligible for mAb-based therapies.
Combinations containing anti-CD38 mAbs
Daratumumab with bortezomib and dexamethasone
In the phase III CASTOR study (NCT02136134) that evaluated daratumumab + bortezomib + dexamethasone compared to bortezomib + dexamethasone demonstrated an improvement in the overall response rate (ORR) of 84% vs 63%, and progression-free survival (PFS) of 17 months vs 7 months (HR, 0.31, respectively). The triplet combination had a higher occurrence of mild myelosuppression and infusion reactions. This regimen was favorable in patients who are lenalidomide-refractory and had minimal neuropathy.
Daratumumab with lenalidomide and dexamethasone
In the phase III POLLUX study (NCT02076009) that assessed daratumumab + lenalidomide + dexamethasone compared with lenalidomide + dexamethasone, an improvement in the ORR (93% vs 76%) and PFS (83% vs 60%) was observed, respectively. The median PFS was 44.5 months in the triplet group vs 17.5 months in the lenalidomide + dexamethasone group (HR, 0.44). The triplet regimen also noted a higher incidence of upper respiratory infections, neutropenia, and diarrhea, but overall, Krishnan highlighted its manageable toxicity profile.1 The triplet combination was preferred among patients who experienced relapse with lenalidomide or bortezomib maintenance, particularly those who were not refractory to full doses of lenalidomide.
Both trials were the first ones assessing the importance of achieving MRD negativity also in the relapsed setting. Click here to read an article about sustained MRD negativity in the POLLUX and CASTOR trials on the Multiple Myeloma Hub.
Exploring the use of another anti-CD38 mAb: Daratumumab vs isatuximab
The IKEMA study (NCT03275285) assessed the efficacy and safety of isatuximab in combination with carfilzomib and dexamethasone vs carfilzomib and dexamethasone alone. The triplet combination demonstrated a median PFS of not reached vs 19.15 months in the doublet arm (p = 0.0007). Safety data showed that up to 3.4% of patients in the triplet regimen experienced fatal treatment-emergent adverse events (TEAEs) vs 3.3% of patients in the doublet arm.
There are studies investigating the efficacy of daratumumab and isatuximab (as previously described above), but there is no data currently available that explores isatuximab treatment after relapse in patients on daratumumab treatment, which is reflective of a real-world scenario. However, in one of the latest reports of the ICARIA-MM trial, which assessed isatuximab (Isa) combined with pomalidomide and dexamethasone (Pd) (Isa-Pd), the investigators reported suboptimal results of the daratumumab single-agent when used as the first subsequent therapy following isatuximab treatment. Interestingly, the effect of sequencing daratumumab after Isa-Pd was not seen when daratumumab was used in combination with a PI, IMiD agent, or alkylating agents.
Lenalidomide-based combinations
There are multiple lenalidomide-based options for early relapsed patients as shown in Table 1. The table shows a high response rate with promising PFS among all treatment arms; click on the hyperlinked regimens depicted on the table to read about each study.
Table 1. Randomized studies with lenalidomide and dexamethasone control arms*
|
CR, complete remission; DRd, daratumumab + lenalidomide + dexamethasone; ERd, elotuzumab + lenalidomide + dexamethasone; HR, hazard ratio; IRd, ixazomib + lenalidomide + dexamethasone; KRd, carfilzomib + lenalidomide + dexamethasone; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; Tx, therapy transplant treatment. |
||||||||||||
|
Characteristic |
Carfilzomib |
Elotuzumab |
Daratumumab |
Ixazomib |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Regimen |
||||||||||||
|
Efficacy |
Tx |
Control |
Tx |
Control |
Tx |
Control |
Tx |
Control |
||||
|
Median follow-up, months |
67.0 |
48.0 |
44.3 |
23.0 |
||||||||
|
ORR, % |
87.1 |
66.7 |
79.0 |
66.0 |
93.0 |
76.0 |
78.3 |
71.5 |
||||
|
CR, % |
32.0 |
9.3 |
5.0 |
9.0 |
55.0 |
23.0 |
12.0 |
7.0 |
||||
|
Median PFS, months |
26.0 |
16.6 |
19.0 |
14.9 |
44.5 |
17.5 |
21.0 |
14.7 |
||||
|
PFS HR (95% CI) |
0.69 |
0.71 |
0.44 |
0.74 |
||||||||
|
Median OS, months |
48.3 |
40.4 |
48.3 |
39.6 |
NR |
NR |
NR |
NR |
||||
|
OS HR (95% CI) |
0.79 |
0.78 |
NR |
NR |
||||||||
Transplant
The IFM 2009 study (NCT01191060) demonstrated prolonged PFS in the arm that received the combination of lenalidomide, bortezomib, and dexamethasone (VRd) with auto-HSCT vs the VRd regimen alone. The overall survival was equivalent in both arms, but it was noticed that 80% of patients in the VRd arm underwent an auto-HSCT at first relapse. Therefore, transplantation should be considered, particularly in eligible patients naïve to auto-HSCT as an early relapse treatment.
Relapse following treatment with lenalidomide and auto-HSCT
Anti-CD38 antibodies are typically favored as first-line treatment in early relapse. The randomized phase III APOLLO trial (NCT03180736) evaluated the efficacy of daratumumab—an anti-CD38 antibody—plus pomalidomide and dexamethasone (DPd) compared with pomalidomide and dexamethasone alone (Pd) in patients with relapsed/refractory (RR) MM (RRMM) who have received ≥1 previous line of therapy, including lenalidomide and a PI. The study used the same class of drug—an IMiD—and swapped lenalidomide with pomalidomide. The results showed a prolonged median PFS in patients in the DPd arm (12.4 months) vs patients who received Pd alone (6.9 months; HR, 0.63; 95% CI, 0.47–0.85; p = 0.0018).
On the other hand, to explore the alternative strategy of changing the drug class from the previous line of therapy (from an IMiD to a PI), the CANDOR study (NCT03158688) compared the efficacy of the daratumumab, carfilzomib and dexamethasone (DKd) combination vs carfilzomib and dexamethasone alone. The median PFS was significantly longer in patients treated with the triplet vs doublet (not reached vs 15.8 months, respectively; HR, 0.63; 95% CI, 0.46–0.85; p = 0.0014). Although the triplet combination was potent, there were safety concerns, particularly for fragile patients, as fatal TEAEs occurred in 9.7% of patients in the DKd arm, and in 5.2% of patients in the Kd arm.
Exploring novel targets
Elotuzumab-based combinations
The phase II ELOQUENT-3 trial investigated pomalidomide and dexamethasone with or without the anti-SLAMF7 mAb, elotuzumab. Results showed a higher ORR (53% vs 26%) and improvement of PFS (10 months vs 5 months; HR, 0.54; p = 0.008) with elotuzumab treatment compared to the doublet, respectively. There is no current consensus on when to use elotuzumab, although it is recommended for patients in the indolent relapse subgroup and elderly patients because it is well tolerated and the cytopenias are more manageable.
The BOSTON trial: Selinexor + bortezomib + dexamethasone
The phase III BOSTON trial (NCT03110562) compared the efficacy of selinexor + bortezomib + dexamethasone (SVd) with bortezomib + dexamethasone (Vd). The median PFS in the SVd arm was 13.93 months vs 9.46 months in the Vd arm (HR, 0.70; 95% CI, 10.53–0.93; p = 0.0066). The ORR was 76.4% vs 62.3% in the SVd vs Vd arm, respectively. The subgroup analysis showed selinexor was clinically beneficial in patients with high-risk cytogenetics (77.3 vs 55.8; p = 0.0008, in the SVd vs Vd groups, respectively). The main safety concern in the trial was thrombocytopenia, as 18% of patients required a thrombopoietin agonist for support. Although selinexor is effective, Amrita Krishnan does not prioritize this regimen in early relapses due to the safety profile.1
Conclusion
In conclusion, there are many options available for patients who relapse. The addition of multiple agents shows an improvement in response rates and length of survival but with the caveat of increased toxicity. The typical approach for patients who relapse whilst on lenalidomide is to switch to pomalidomide and add an anti-CD38 antibody, particularly daratumumab; however, many questions, such as “Does switching drug class translate to clinical benefit?”, are yet to be answered.
As the treatment landscape continues to expand, a careful consideration of factors that may influence patient outcomes is needed, including prior therapies, the aggressiveness of relapse, comorbidities, and access to care. It is expected that immune-based therapies will play an increasingly central role in the RRMM setting due to their promising efficacy in high-risk patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

