All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
TOURMALINE-MM2 trial: Ixazomib with Rd versus Rd in newly diagnosed patients with MM
Transplant ineligible patients who are diagnosed with multiple myeloma (MM) are a diverse group; some are fit while some are very frail, which means one treatment does not fit all. In particular, a patient’s ability to travel frequently to the clinic for treatment is variable. In this scenario, an oral proteasome inhibitor like ixazomib could reduce the treatment burden and improve therapy tolerance.
At the Eighth Annual Meeting of the Society of Hematologic Oncology (SOHO) and the 6th World Congress on Controversies in Multiple Myeloma (COMy), Thierry Facon presented the results of the phase III TOURMALINE-MM2 trial (NCT01850524) that tested the oral triplet combination of ixazomib (ixa), lenalidomide (R, len), and dexamethasone (d, dex).1,2
Study design
The study included 705 patients with newly diagnosed (ND)MM.
Inclusion criteria:
- Adult patients with measurable NDMM diagnosed according to the International Myeloma Working Group criteria
- Transplant-ineligible but suitable for treatment with len and dex
- Eastern Cooperative Oncology Group performance score 0−2
- Sufficient hepatic, hematological, and renal function (creatinine clearance ≥ 30 ml/min)
Exclusion criteria:
- Previously treated for MM
- Unmanaged cardiovascular condition
- Unable to tolerate/receive prophylactic treatment for thromboembolisms
- Any major surgery, severe infection, or localized radiation treatment 2 weeks prior to randomization
- Peripheral neuropathy Grade ≥ 2 or Grade 1 with pain
Patients were randomized 1:1 to receive Rd with a placebo or with ixa as shown in Figure 1.
Figure 1. Study design and dosing1
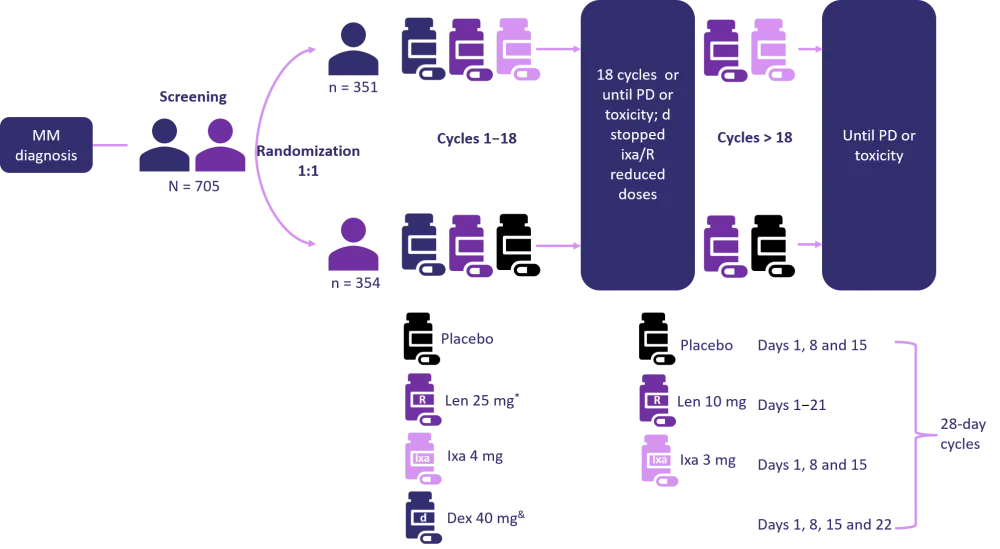
R, lenalidomide, PD, disease progression, MM multiple myeloma.
*10 mg in patients with renal impairment.
&Patients ≥ 75 years.
Patient characteristics were similar between the two treatment arms, with over 40% of patients being 75 years or older (Table 1). Almost 40% of patients had high-risk cytogenetics, and 58% had creatinine clearance greater than 60 ml/min.
Table 1. Patient baseline characteristics1
|
CrCl, creatinine clearance; d, dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance score; ISS, International Staging System; Ixa, ixazomib; R, lenalidomide. |
|||
|
Characteristic |
Ixa-Rd (n = 351) |
Placebo-Rd (n = 354) |
|
|---|---|---|---|
|
Median age (range), years |
73 (48−90) |
74 (48−88) |
|
|
Age ≥ 75 years, % |
43 |
44 |
|
|
ECOG PS % |
0 |
31 |
30 |
|
1 |
52 |
56 |
|
|
2 |
17 |
14 |
|
|
ISS stage at entry, % |
I |
49 |
43 |
|
II |
35 |
40 |
|
|
III |
16 |
17 |
|
|
Expanded high-risk cytogenetics*, % |
38 |
41 |
|
|
CrCl > 60 ml/min, % |
58 |
58 |
|
The primary endpoint was progression-free survival (PFS).
Time to progression (TTP) was also assessed and differs from PFS in that it only counts as disease progression, death was not counted as an event.
The secondary endpoints were:
- Overall survival (OS)
- Complete response (CR) rate
Key findings
- The median follow-up for PFS was 53.3 months for the ixa-Rd arm compared with 55.8 months for the control arm. In both groups, only 54% of patients entered Cycle 19.
- There was a significantly increased TTP in the ixa-Rd arm compared with the placebo arm, 45.8 months compared with 26.8 months, respectively (HR 0.738 [95% CI, 0.589−0.925]; p = 0.008).
- Median PFS for ixa-Rd was 35.3 months vs 21.8 for the placebo (HR 0.830 [95% CI, 0.676−1.018]; p = 0.073). The study did not meet its primary endpoint of PFS. However, there was a positive trend in the ixa arm in PFS. When the overall group was split into certain subgroups, as shown in Table 2, a PFS benefit was seen vs Rd.
Table 2. PFS in specific subgroups of note1
|
d, dexamethasone; ixa, ixazomib; ISS, International Staging System; PFS, progression-free survival; R, lenalidomide. |
||||||
|
Subgroup |
Event n/N |
Median PFS, months |
HR |
95% CI |
||
|---|---|---|---|---|---|---|
|
Ixa-Rd |
Placebo-Rd |
Ixa-Rd |
Placebo-Rd |
|||
|
≥ 75 years (n=308) |
94/198 |
117/199 |
27.9 |
20.5 |
0.871 |
0.640−1.186 |
|
ISS stage III at screening (n = 123) |
33/59 |
47/64 |
27.9 |
16.1 |
0.736 |
0.466−1.163 |
|
Expanded high-risk cytogenetics (n=280) |
80/134 |
104/146 |
23.8 |
18.0 |
0.690 |
0.506−0.941 |
|
≤ 60 ml/min (n = 298) |
65/148 |
100/150 |
40.0 |
19.4 |
0.625 |
0.450−0.869 |
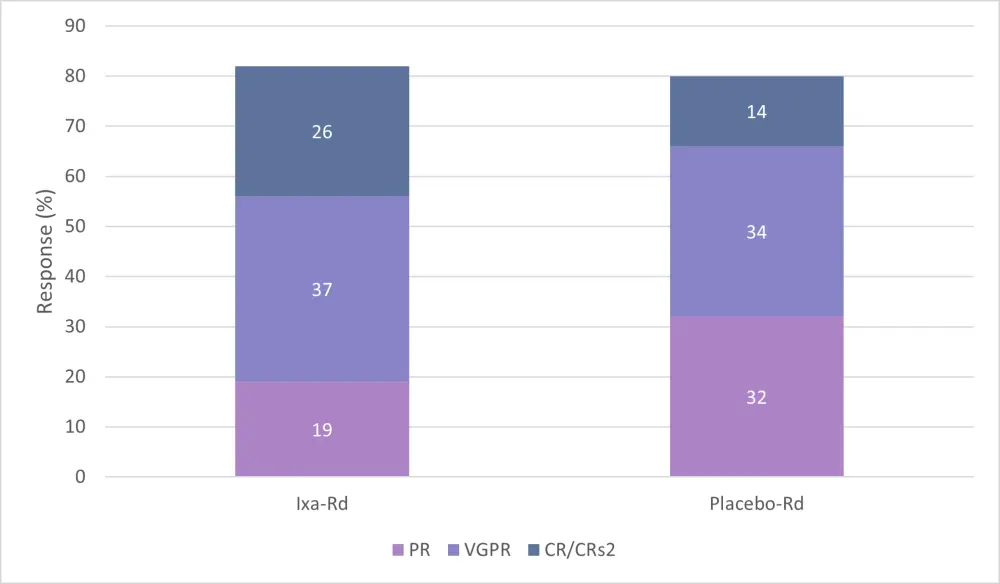
A CR was seen in 26% of patients in the ixa-Rd group, with an overall response rate (ORR) of 82.1% (Figure 2). In the placebo arm, a CR of 14% (p < 0.001) and an ORR of 79.7% were reached, which was not significantly different from the ixa-Rd arm. The difference in the percentage of very good partial responses achieved between groups was significant (p < 0.001).
Figure 2. Response rate between treatment arms1
The median follow-up for OS was similar in the two treatment arms, 57.8 months compared with 58.6 months for the ixa-Rd group vs the placebo group. In both treatment arms, the median OS was not reached but to date, both curves practically overlap (HR 0.998 [95% CI, 0.790−1.261]; p = 0.988).
Safety
Treatment-emergent adverse events (TEAEs).
In the study, all patients had a TEAE, with 88.1% of ixa-Rd patients experiencing Grade ≥ 3 compared with 81.4% in the placebo arm, as shown in Figure 3.
Figure 3. Safety profile of ixa-Rd vs placebo-Rd1
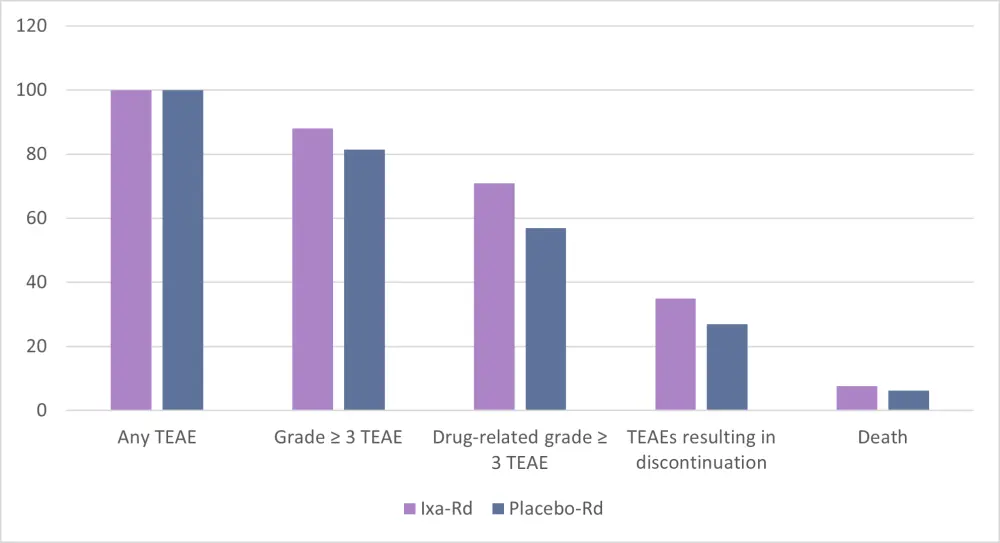
The most frequent TEAEs were diarrhea 61.0% vs 46.1%, rash 56.2% vs 37.2%, and peripheral edema 48.6% vs 33.5% for the ixa-Rd group compared with the placebo cohort, respectively. In terms of Grade ≥ 3 TEAEs, neutropenia, anemia, and thrombocytopenia were the most common. Of note, peripheral neuropathy was reported in 33.9% of patients with ixa-Rd (vs 27.5 with Rd), but the vast majority were considered of Grade 1–2.
Conclusions
While the study did not meet its primary endpoint, positive results were seen in the percentage of patients achieving CR and the time to progression in the ixa-Rd arm. The safety profile was consistent with previous reports and was generally tolerated in this elderly patient population. This oral treatment combination could reduce the treatment burden for transplant-ineligible patients with NDMM.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

