All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Updated results of the ICARIA-MM trial of isatuximab, pomalidomide, and dexamethasone in patients with relapsed/refractory multiple myeloma
Isatuximab (Isa) is an anti-CD38 antibody approved in combination with pomalidomide and dexamethasone (Pd) for patients with relapsed/refractory multiple myeloma (R/R MM). This approval was based on the initial results of the ICARIA-MM trial (NCT02990338).
During the 26th Congress of the European Hematology Association (EHA2021), Aurore Perrot gave an update on this trial with a longer follow-up, and the Multiple Myeloma Hub is happy to provide a summary.1 This prespecified second interim analysis was later published in The Lancet Oncology by Paul G. Richardson et al.2
Study design
The ICARIA-MM trial is an open-label, multicenter, randomized phase III study evaluating the efficacy of Isa-Pd compared with Pd in terms of progression-free survival (PFS), in patients with R/R MM. The Multiple Myeloma Hub has previously reported on this trial; the first interim results (cutoff date in October 2018) are summarized here, where you can read more details on the study design and patient eligibility.
This second interim analysis had a cutoff date of October 2020 and a median follow-up time of 35.3 months, and it reports the following outcomes:
- Time to next treatment (TTNT)
- Overall survival (OS)
- Time from randomization to disease progression on first subsequent therapy or death (PFS2)
- Safety
Results
Survival outcomes
The survival outcomes for the second interim analysis showed that Isa-Pd significantly increased PFS, PFS2, OS, and TTNT compared with Pd alone (Table 1).
Table 1. Survival outcomes*
|
d, Dexamethasone; HR, hazard ratio; Isa, isatuximab; P, pomalidomide; PFS, progression-free survival; OS, overall survival; TTNT, time to next treatment. |
||||
|
Outcome |
Isa-Pd |
Pd |
HR |
p value |
|---|---|---|---|---|
|
Median PFS† |
11.1 |
5.9 |
0.60 |
< 0.0001 |
|
Median TTNT |
15.5 |
8.9 |
0.56 |
< 0.0001 |
|
Median PFS2 |
17.5 |
12.9 |
0.76 |
= 0.0202 |
|
Median OS |
24.6 |
17.7 |
0.76 |
= 0.028 |
The overall response rate (ORR) at the first interim analysis is compared with the second in Figure 1. The ORR in the Isa-Pd group was 63.0% at both time points, whereas in the Pd group, there was a slight increase from 32.0% to 33.3%. However, when ≥ very good partial response was looked at, the Isa-Pd group saw an increase from 33.8% to 38.3%. In the Pd arm, ≥ very good partial response also increased from 7.2% in 2018 to 10.5%. Therefore, responses were seen to deepen with continuous therapy in the 2 years between analyses.
Figure 1. Difference in the depth of response between the two treatment arms at two timepoints*
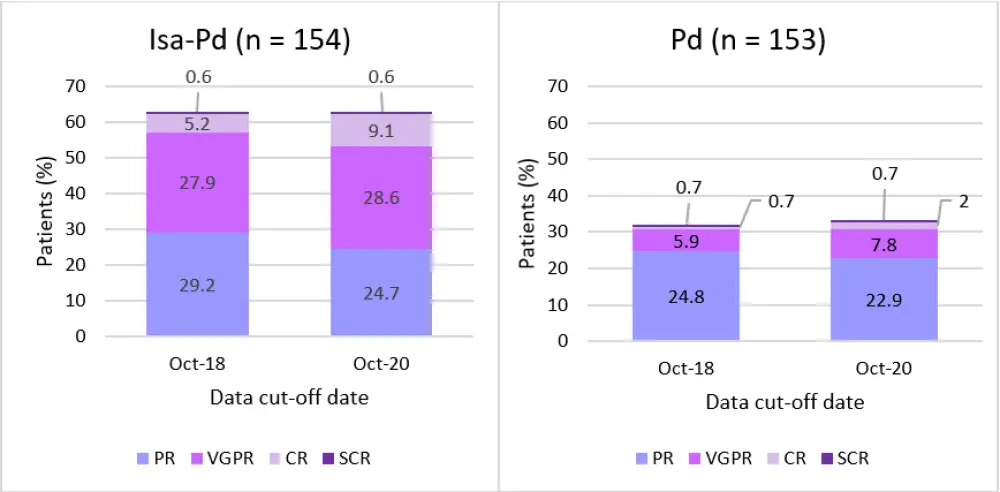
CR, complete response; Isa, Isatuximab; Pd, pomalidomide and dexamethasone; PR, partial response; SCR, stringent complete response; VGPR, very good partial response.
*Adapted from Perrot et al. and Richardson et al.1, 2
Treatment duration
Median treatment duration was almost twice as long in the Isa-Pd arm (47.6 weeks) compared with the Pd arm (24.0 weeks) (Table 2). In addition, patients in the Isa-Pd arm had almost double the median number of cycles received compared with the Pd arm.
Table 2. Exposure to study treatments*
|
d, dexamethasone; Isa, isatuximab; P, pomalidomide. |
||
|
Safety population |
Isa-Pd |
Pd |
|---|---|---|
|
Cumulative exposure to treatment, patient-years |
184.94 |
124.50 |
|
Median treatment duration, weeks (range) |
47.6 (1.3−171.6) |
24.0 |
|
Relative dose intensity, median % (range) |
||
|
Isatuximab |
91.06 (19.7−111.1) |
— |
|
Pomalidomide |
81.86 (22.9−103.7) |
91.46 (37.2−118.5) |
|
Dexamethasone |
85.19 (13.6−130.0) |
95.68 (30.3−300.0) |
|
Total number of cycles |
2,296 |
1,590 |
|
Median number of cycles/patient (range) |
11 (1− 42) |
6 (1− 40) |
Effect of subsequent daratumumab (dara) therapy
Out of the 92 patients treated with Isa-Pd, only 23.9% were subsequently treated with daratumumab, whereas 58.2% of the patients in the Pd arm (n = 110) moved on to daratumumab therapy after.
When daratumumab was used as a first subsequent therapy following isatuximab treatment, the median PFS was only 2.2 months (n = 9) vs 5.1 months in the Pd arm (n = 46). When a non-daratumumab first subsequent treatment was used, the median PFS between groups was more similar with Isa-Pd at 4.2 months (n = 82) and Pd at 5.0 months (n = 64), showing that the survival benefit of using daratumumab treatment was reduced when it was used following isatuximab, which is another CD38 antibody.
Interestingly, the effect of sequencing of daratumumab after Isa-Pd was not seen when daratumumab was used in combination with a proteasome inhibitor, immunomodulatory agent or alkylating agents, whereas when daratumumab was used as a monotherapy or with steroids, a pronounced difference in ORR was seen (Table 3).
Table 3. ORR for patients treated with daratumumab as first subsequent therapy*
|
d, dexamethasone; dara, daratumumab; IMiD, immunomodulatory drugs; Isa, isatuximab; ORR, overall response rate; P, pomalidomide; PI, proteasome inhibitor. |
||
|
Dara regimen |
Isa-Pd |
Pd |
|---|---|---|
|
Monotherapy or combined with steroids, % |
14.3 |
37.9 |
|
Combined with PI, IMiD, or alkylating agent, % |
30.8 |
31.8 |
Safety
Between the Isa-Pd and Pd arms, the percentage of treatment-emergent adverse events (TEAEs) that lead to a fatal outcome were similar at 9.2% and 10.1%, respectively. In addition, the percentage of TEAEs leading to treatment discontinuation was comparable at 11.8% in the Isa-Pd arm and 14.1% in the Pd arm (Table 4).
Table 4. Safety summary data*
|
d, dexamethasone; Isa, isatuximab; P, pomalidomide; TEAE, treatment-related adverse events; SAE, severe adverse event. |
||
|
Patients (%) |
Isa-Pd |
Pd |
|---|---|---|
|
Any TEAE |
99.3 |
98.0 |
|
Any grade ≥ 3 TEAE |
90.8 |
75.2 |
|
Treatment-related grade ≥ 3 TEAE |
75.7 |
50.3 |
|
Any Grade 5 TEAE† |
9.2 |
10.1 |
|
Any SAE |
73.0 |
60.4 |
|
Any TEAE leading to definitive discontinuation |
11.8 |
14.1 |
The investigators reported that no new safety signals were identified since the first interim analysis. The breakdown of hematologic and nonhematologic TEAEs is shown in Table 5. Pneumonia was the most common nonhematologic Grade ≥3 TEAE in both treatment arms, whereas neutropenia was the most common hematologic TEAE and even reached 62.5% Grade 4 in the Isa-Pd arm. Of note, granulocyte colony-stimulating factor was administered as supportive treatment in 72% and 54% of patients in the experimental and control arm, respectively.
Table 5. TEAEs for both treatment arms*
|
d, dexamethasone; Isa, isatuximab; P, pomalidomide; TEAE, treatment-emergent adverse event; URTI, upper respiratory tract infection. |
||||
|
Nonhematologic TEAEs occurring in ≥ 20% patients in either arm, % |
Isa-Pd (n = 152) |
Pd (n = 149) |
||
|---|---|---|---|---|
|
All grades |
Grades ≥ 3 |
All grades |
Grades ≥ 3 |
|
|
Infusion reaction |
37.5 |
2.6 |
1.3 |
0 |
|
URTI |
34.2 |
3.3 |
19.5 |
1.3 |
|
Diarrhea |
30.3 |
2.0 |
22.1 |
1.3 |
|
Pneumonia |
27.6 |
23.0 |
25.5 |
20.8 |
|
Bronchitis |
27.0 |
4.6 |
11.4 |
0.7 |
|
Fatigue |
19.7 |
3.9 |
21.5 |
0 |
|
Constipation |
16.4 |
0 |
20.1 |
0 |
|
Hematologic abnormalities from lab values, % |
Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
|
Anemia |
34.9 |
0 |
28.6 |
0 |
|
Neutropenia |
22.4 |
62.5 |
38.8 |
32.7 |
|
Thrombocytopenia |
14.5 |
19.7 |
10.2 |
15.0 |
Conclusions
The ICARIA-MM study met its primary objective and demonstrated significantly improved PFS in the Isa-Pd arm for patients with R/R MM. There was also a significant increase in TTNT and OS survival for Isa-Pd compared with Pd. The subanalysis on subsequent therapy showed that daratumumab monotherapy after Isa-Pd was less effective than when daratumumab was used after Pd. However, this reduction in efficacy was not seen when daratumumab was used in combination therapy after Isa-Pd treatment. The safety profile for Isa-Pd remained consistent with the first interim analysis. Overall, these results show that Isa-Pd continues to be a standard of care for patients with R/R MM.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

