All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
APOLLO trial results: Addition of daratumumab to pomalidomide + dexamethasone in relapsed/refractory multiple myeloma
The immunomodulatory agent, pomalidomide, combined with dexamethasone, is effective in patients with multiple myeloma refractory to lenalidomide and proteasome inhibitors (PI).1 The addition of CD38-targeted monoclonal antibody (mAb) therapy to this regimen has been shown to further improve outcomes for patients with relapsed/refractory disease,1,2 and positive trial results for the anti-CD38 mAb, isatuximab, plus pomalidomide and dexamethasone has led to European approval of this combination, but the regimen requires intravenous administration.
The randomized phase III APOLLO study (NCT03180736) was designed to evaluate the safety and efficacy of the anti-CD38 mAb, daratumumab, plus pomalidomide and dexamethasone (D-Pd) versus pomalidomide and dexamethasone alone (Pd) in patients with relapsed and/or refractory multiple myeloma (RRMM) who have received ≥ 1 prior line of therapy, including lenalidomide and a PI. D-Pd is currently approved in the U.S. for patients with RRMM with ≥ 2 previous lines of therapy, including lenalidomide and a PI, and requests for approval of subcutaneous (SC) administration of daratumumab with Pd for RRMM were recently submitted to the European Medicines Agency and U.S. Food and Drug Administration (read more here). Positive results from the APOLLO study formed the basis of this request; here, we summarize the primary analysis of the trial presented by Meletios Dimopoulos at the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.3
Study design
The study included 304 patients with RRMM.
Key eligibility criteria:
- ≥ 1 prior line of therapy with lenalidomide and a PI
- Eastern Cooperative Oncology Group performance status ≤ 2
- Creatinine clearance ≥ 30 mL/min
Patients were randomized 1:1 to receive either D-Pd or Pd in 28-day cycles until progressive disease or unacceptable toxicity. The dosing schedule is shown in Figure 1; for patients older than 75, dexamethasone was adjusted to 20 mg.
Figure 1. Study design and dosing schedule3
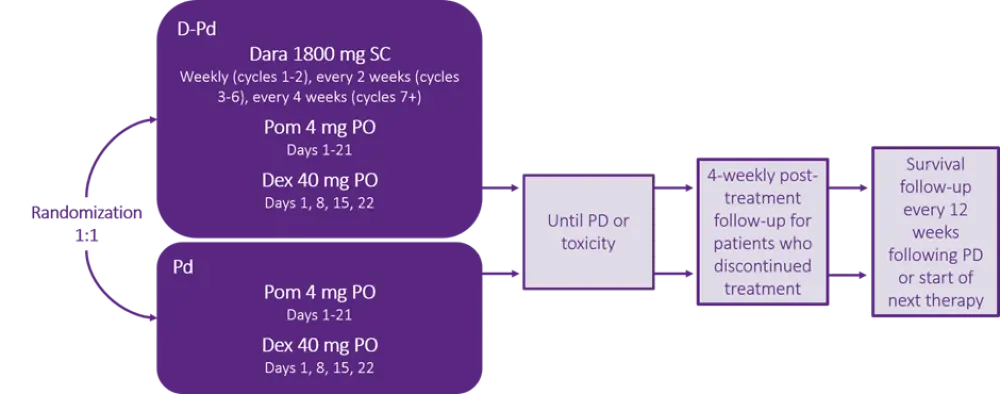
D-Pd, daratumumab, pomalidomide, and dexamethasone; Dara, daratumumab; Dex, dexamethasone; Pd, pomalidomide and dexamethasone; PD, progressive disease; PO, oral; Pom, pomalidomide; SC, subcutaneous.
Primary endpoint:
- Progression-free survival (PFS)
Secondary endpoints:
- Overall response rate (ORR), including very good partial response (VGPR) and complete response (CR) rates
- Minimal residual disease (MRD), assessed by next-generation sequencing of bone marrow aspirates
- Overall survival
- Time to and duration of response
- Time to next therapy
- Safety
- Health-related quality of life
Results
Selected demographic and baseline disease characteristics for the patient cohort are summarized in Table 1. Overall, baseline characteristics were well balanced between treatment arms.
Table 1. Selected patient characteristics3
|
Characteristic |
D-Pd |
Pd |
|
|
ASCT, autologous stem cell transplant; D-Pd, daratumumab, pomalidomide, and dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, international staging system; MM, multiple myeloma; Pd, pomalidomide and dexamethasone; PI, proteasome inhibitor. |
|||
|
Median age (range), years |
67 (42–86) |
68 (35–90) |
|
|
Age ≥ 75 years, % |
17 |
20 |
|
|
ECOG PS, % |
0 |
60 |
50 |
|
1 |
36 |
37 |
|
|
2 |
4 |
12 |
|
|
ISS disease stage, % |
I |
45 |
45 |
|
II |
33 |
33 |
|
|
III |
22 |
22 |
|
|
Type of MM, % |
IgG |
55 |
57 |
|
IgA |
23 |
20 |
|
|
Light chain |
17 |
20 |
|
|
High risk cytogenetics, % |
38 |
32 |
|
|
Prior lines of therapy, % |
1 |
11 |
12 |
|
|
2-3 |
75 |
74 |
|
|
≥ 4 |
14 |
14 |
|
Prior ASCT, % |
60 |
53 |
|
|
Disease refractory to, % |
Lenalidomide |
79 |
80 |
|
|
PI |
47 |
49 |
|
|
PI + lenalidomide |
42 |
42 |
- Overall, 95% of patients in the D-Pd arm started treatment with SC daratumumab.
- Treatment discontinuation occurred in 60% of patients receiving D-Pd and in 78% of patients receiving Pd, most commonly due to progressive disease (44% and 58%, respectively). Only 5% of patients receiving D-Pd discontinued due to adverse events or physician decision.
- Median study duration was longer for the D-Pd arm (11.5 months) compared with the Pd arm (6.6 months).
Efficacy – PFS
- Median follow-up for PFS was 16.9 months.
- Median PFS for patients treated with D-Pd was significantly longer (12.4 months) than for patients treated with Pd (6.5 months; HR, 0.63; 95% CI, 0.47–0.85; p = 0.0018). The 12-month PFS rates were 52% for D-Pd vs 35% for Pd.
- For patients refractory to lenalidomide, median PFS rates were 9.9 months and 6.5 months for the D-Pd and Pd arms, respectively.
- The impact of treatment on PFS across different pre-specified subgroups was generally consistent, as shown in Table 2.
Table 2. Progression-free survival in pre-specified subgroups of note3
|
Subgroup |
No. of progression events or deaths/total |
HR (95% CI) |
||
|
D-Pd |
Pd |
|||
|
D-Pd, daratumumab, pomalidomide, and dexamethasone; Pd, pomalidomide and dexamethasone. |
||||
|
Age ≥ 65 years |
48/88 |
65/93 |
0.55 (0.38–0.81) |
|
|
ISS disease stage III |
21/33 |
27/33 |
0.75 (0.42–1.32) |
|
|
High risk cytogenetics |
28/39 |
26/35 |
0.85 (0.49–1.44) |
|
|
Number of prior therapies |
1 |
9/16 |
12/18 |
0.70 (0.30–1.67) |
|
2-3 |
65/144 |
79/113 |
0.66 (0.48–0.92) |
|
|
≥ 4 |
10/21 |
15/22 |
0.40 (0.18–0.90) |
|
|
Refractory to lenalidomide |
76/120 |
89/122 |
0.66 (0.49–0.90) |
|
Efficacy – depth of response
Hematologic response rates are shown in Figure 2. ORR, ≥ VGPR, ≥ CR, and MRD-negativity rates were significantly higher for patients receiving D-Pd vs Pd:
- ORR: 69% vs 46% (odds ratio, 2.68; 95% CI, 1.65–4.35; p < 0.0001)
- ≥ VGPR: 51% vs 20% (p < 0.0001)
- ≥ CR: 25% vs 4% (p < 0.0001)
- MRD-negativity rate: 8.6% vs 2%; 4.3-fold increase (p = 0.0102)
Figure 2. Comparison of hematologic response rates between treatment arms3
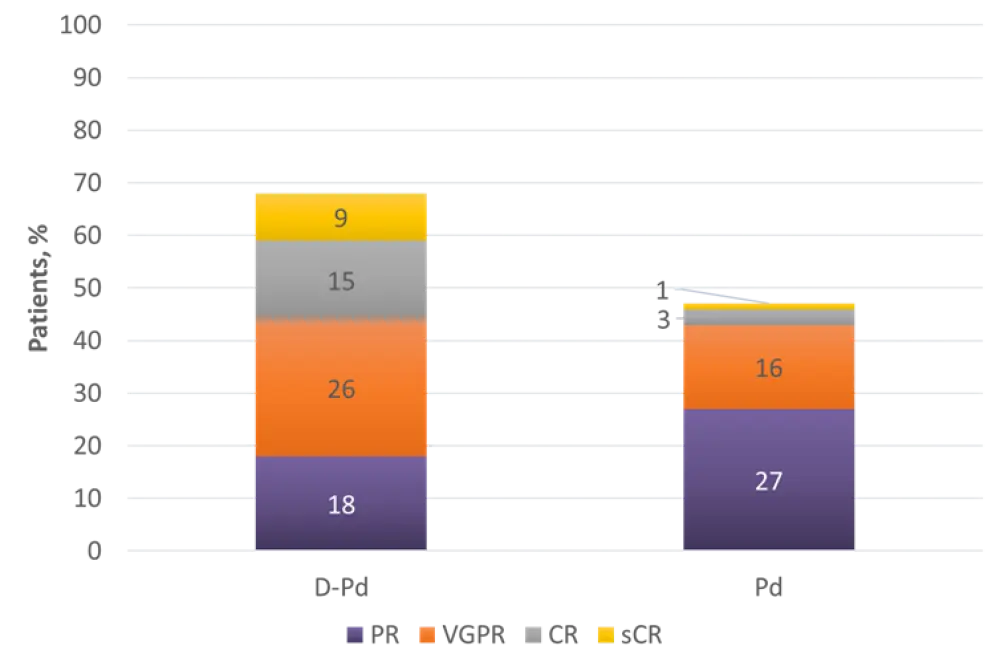
CR, complete response; D-Pd, daratumumab, pomalidomide, and dexamethasone; Pd, pomalidomide and dexamethasone; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
Adverse events
The most common Grade 3/4 treatment-emergent adverse events (TEAEs) are shown in Table 3. The safety profile was consistent with the known profiles of SC daratumumab and Pd alone; no new safety concerns were identified. Safety results of note were as follows:
- Infusion-related reactions were reported in 5% of D-Pd-treated patients; all were Grade 1 or 2.
- In total, 2% of patients had local injection-site reactions after SC daratumumab administration; all were Grade 1.
- The most common serious TEAEs were pneumonia (15% for D-Pd vs 8% for Pd) and lower respiratory tract infection (12% for D-Pd vs 9% for Pd).
- The incidence of TEAEs leading to treatment discontinuation was similar in both arms (2% for D-Pd vs 3% for Pd).
- TEAEs leading to death occurred in 7% of patients for each arm.
Table 3. Most common Grade 3/4 TEAEs3
|
Most common Grade 3/4 TEAEs, % |
D-Pd |
Pd |
|
D-Pd, daratumumab, pomalidomide and dexamethasone; Pd, pomalidomide and dexamethasone; TEAE, treatment-emergent adverse event. |
||
|
Hematologic |
||
|
Neutropenia |
68 |
51 |
|
Anemia |
17 |
21 |
|
Thrombocytopenia |
17 |
18 |
|
Leukopenia |
17 |
5 |
|
Lymphopenia |
12 |
3 |
|
Febrile neutropenia |
9 |
3 |
|
Nonhematologic |
||
|
Infections |
28 |
23 |
|
Fatigue |
8 |
5 |
|
Asthenia |
5 |
1 |
|
Diarrhea |
5 |
1 |
|
Hyperglycemia |
5 |
5 |
Conclusion
With a 37% decreased risk of progression or death and achievement of deeper hematologic and molecular responses, this primary analysis shows that the addition of SC daratumumab to Pd significantly improves treatment efficacy for patients with RRMM, compared with Pd alone. Moreover, no new safety concerns were identified, and this mAb/immunomodulatory drug combination may therefore provide a convenient and well-tolerated treatment option for patients who have progressed following exposure to both lenalidomide and a PI.
The APOLLO trial is ongoing, and overall survival data are anticipated to be reported in the latter part of 2021 or early 2022.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

