All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, the results of a long-term follow-up analysis from the IFM 2009 trial were presented and highlighted by the medical community for their relevance to clinical practice. Here, we are pleased to summarize the key points.1
The IFM 2009 trial (NCT01191060 and NCT03679351) is a randomized, multicenter, prospective trial comparing bortezomib, lenalidomide, and dexamethasone (VRd) combination alone or followed by high-dose melphalan plus autologous stem cell transplantation (ASCT) in patients with newly diagnosed multiple myeloma (NDMM). The second interim analysis was carried out in 2015 with a median follow-up of 44 months, where median progression-free survival (PFS) was 50 months in the ASCT arm and 36 months in the VRd-only arm (HR, 0.65; 95% CI, 0.53–0.8; p < 0.001).2
Study design
The study design is shown in Figure 1. The VRd regimen was given as below in 21-day cycles:
- Lenalidomide: 25 mg/day (Days 1–14)
- Bortezomib: 1.3 mg/m2 (Days 1, 4, 8, and 11)
- Dexamethasone: 20 mg/day (Days 1, 2, 4, 5, 8, 9, 11, and 12)
Lenalidomide maintenance was given for 1 year only and then discontinued. Researchers at the Dana Farber Cancer Institute (DFCI) are conducting a parallel trial in the US (DFCI 10-106, NCT01208662), with the only difference being that lenalidomide maintenance is given until progression. The DFCI trial started later than IFM 2009, and no data have been reported yet. Results from both trials will be analyzed together in the future, adding another 700 patients with NDMM. Hence, as a part of this international collaboration, the IFM 2009 trial is usually referred to as the IFM/DFCI 2009 trial.
Figure 1. IFM 2009 study design1
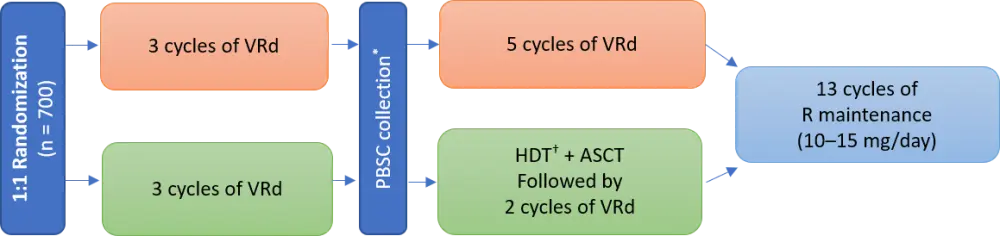
*Cyclophosphamide 3 g/m2 + G-CSF 10 µg/kg/day.
†High-dose melphalan 200 mg/m2.
Primary endpoint was PFS, and secondary endpoints included overall response rate, minimal residual disease (MRD), time to progression, overall survival (OS), and toxicity.
Baseline characteristics were well balanced between the two treatment arms. The number of patients included in the study was 700 (n = 350 in each arm), with a median age of 59 years. Most patients (83%) had standard-risk disease versus high-risk disease in 17% of patients. High-risk disease was defined by the detection of t(4;14), del(17p), or t(14;16). The proportion of patients with International Staging System (ISS) stage III disease was 18%.
Results
This analysis provided data from a median follow-up of 89.8 months. More patients in the VRd-only arm relapsed and needed a second-line treatment compared with the transplantation arm (see Table 1). The majority of patients in the VRd-only arm underwent ASCT at first relapse. Other frequent treatment options included pomalidomide-, carfilzomib-, and daratumumab-based therapies, anthracyclines, alkylating agents, and bendamustine.
There was no difference in PFS2 (time to progression on second-line therapy) or 8-year OS among both arms, which may be attributed to salvage therapy (mainly pomalidomide-based therapy and ASCT) or the lack of continuous maintenance therapy until relapse. However, a higher statistically significant proportion of patients from the transplantation arm achieved MRD negativity (by flow cytometry) at a sensitivity level of 1 in 106 cells.
Subgroup analysis suggested that the transplant approach provided a higher benefit than VRd alone in all subgroups, particularly in patients with high-risk disease or ISS stage III.
Table 1. Analysis results1
|
Outcome |
VRd-only arm |
Transplantation arm |
p value |
|---|---|---|---|
|
ASCT, autologous stem cell transplantation; MRD, minimal residual disease; NR, not reached; OS, overall survival; PFS, progression-free survival; PFS2, PFS on second-line therapy; VRd, bortezomib, lenalidomide, and dexamethasone. |
|||
|
Median PFS, months |
35 |
47.3 |
― |
|
Progressed disease, n (%) |
270 (77.1) |
227 (64.9) |
― |
|
Need for second-line treatment, n |
262 |
217 |
― |
|
Median PFS2*, months |
95.3 |
NR |
0.75 |
|
8-year OS†, % |
60.2 |
62.2 |
0.85 |
|
MRD negativity, % |
20.4 |
29.8 |
0.01 |
Tolerability
Second primary malignancies were recorded based on 8-year follow-up data, and there was no difference between the two arms (Table 2). High-dose melphalan was associated with a higher rate of hematological toxicity in line with historical data. There were significant improvements in both arms in global health-related quality of life, physical and role functioning scores.
Table 2. Second primary malignancies among arms1
|
SPM, % |
VRd-only arm |
Transplantation arm |
|---|---|---|
|
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; SPM, second primary malignancy; VRd, bortezomib, lenalidomide, and dexamethasone. |
||
|
Patients with ≥ 1 invasive SPM |
7.7 |
9.7 |
|
MDS/AML |
0.9 |
1.7 |
|
Breast cancer |
0.9 |
1.1 |
|
Colon cancer |
0.9 |
1.1 |
|
Lung neoplasm |
0.9 |
0.6 |
|
Prostate cancer |
0.9 |
0.9 |
|
Malignant melanoma |
0.9 |
0 |
Conclusion
In this long-term analysis of the IFM 2009 trial, VRd ± ASCT, followed by lenalidomide maintenance, was associated with a survival > 60% 8 years after treatment initiation in NDMM.
Induction therapy with VRd and transplantation is considered the first choice for the treatment of NDMM and is associated with a reduction in risk of relapse or death of 35%, a higher MRD negativity rate, and acceptable toxicity after a follow-up of 8 years. However, this trial also demonstrated that delayed ASCT could also be an option for patients with myeloma under exceptional circumstances, through the effective salvage treatment alternatives available today.
Using transplant combined with the most effective quadruplets and maintenance therapy until progression was suggested as the next best approach to further improve these outcomes in patients with NDMM.
Expert Opinion Video
How does the IFM2009 trial impact the current treatment of transplant-eligible patients?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Philippe Moreau
Philippe Moreau