All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Symposium | Practical considerations when sequencing novel agents and managing AEs with BCMA-directed therapies
Do you know... When sequencing BCMA-directed therapies for RRMM, which practical consideration may favor selecting an option that can be administered in the community setting?
_On December 17, 2025, the Multiple Myeloma Hub held a virtual symposium, titled Integrating novel BCMA-directed therapies into clinical practice: Insights from real-world experience. During the symposium, Hermann Einsele, Julius-Maximilians-Universität Würzburg, DE, chaired a panel discussion focusing on key considerations when sequencing novel agents and managing adverse events with B-cell maturation antigen (BCMA)-directed therapies in relapsed/refractory multiple myeloma (RRMM).
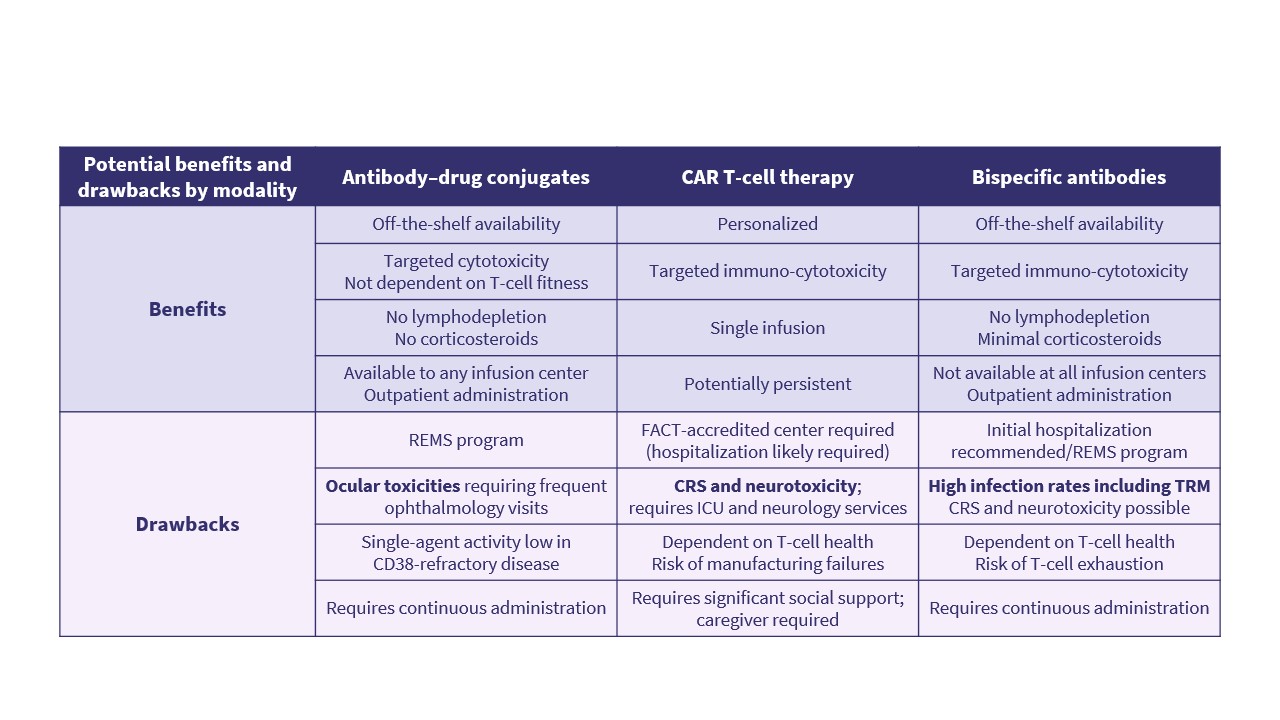
Einsele presented mechanisms of resistance with BCMA-targeted therapies; the impact of prior exposure to BCMA-directed therapies; the main differences between BCMA-directed antibody–drug conjugates (ADCs), bispecific antibodies, and chimeric antigen receptor (CAR) T-cell therapies in terms of access, logistics and toxicity profiles (Figure 1); and how dosing schedules can be adapted to optimize efficacy while limiting toxicity.
Figure 1. Key differences between BCMA-directed therapy modalities*
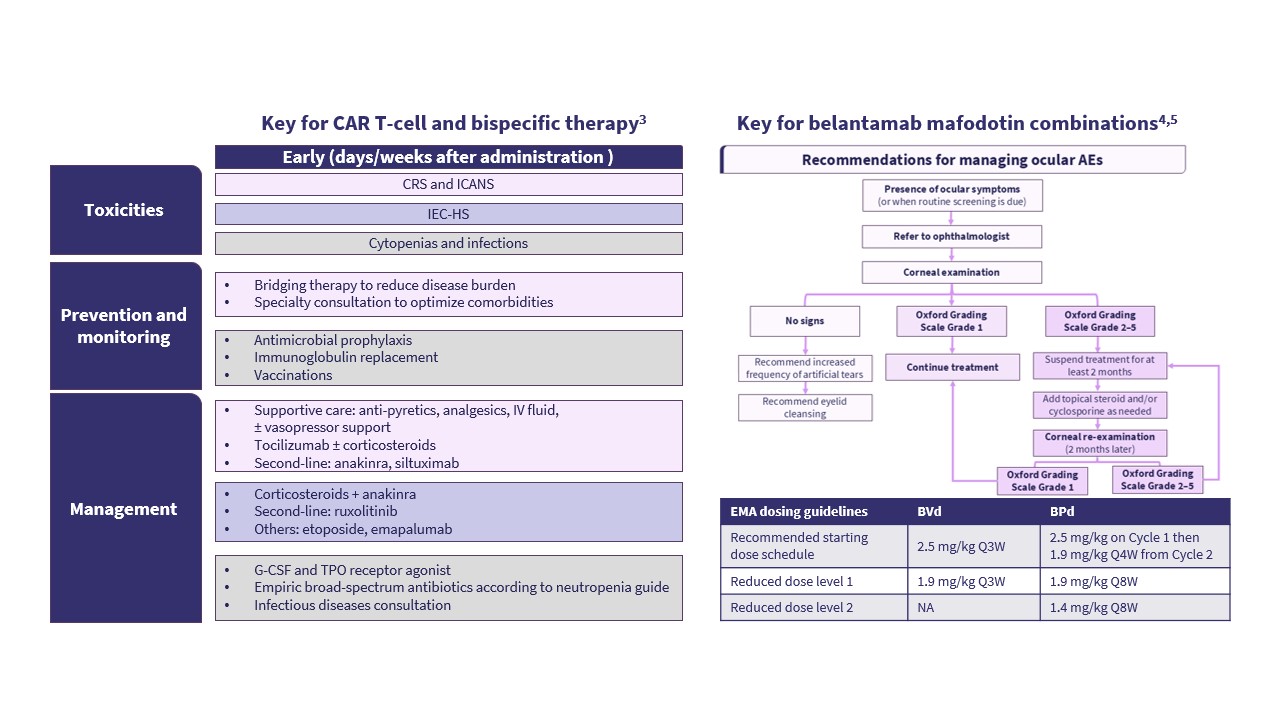
He went on to share a case study of a 67-year-old female patient with RRMM and standard-risk cytogenetics who had received four prior lines of therapy, including immunomodulatory drugs, protease inhibitors, and anti-CD38 antibodies. The patient was in clinical relapse and ineligible for CAR T-cell therapy. The case study prompted a discussion on what should be the preferred next lines of therapy for this patient and concluded with a discussion on the optimal management of ocular events in a patient receiving belantamab mafodotin (Figure 2).
Symposium | Practical considerations when sequencing novel agents and managing AEs with BCMA-DTs
Figure 2. Strategies for managing AEs*
Key points
Mechanisms of resistance to BCMA-targeted therapies include mechanisms related to the tumor microenvironment, tumor intrinsics mechanisms (e.g. γ-secretase cleaving BCMA from the myeloma cell, loss of expression of BCMA on myeloma cells through downregulation of gene expression, and increased expression of the inhibitory ligand, programmed death-ligand 1, on the surface of myeloma cells), and tumor extrinsic mechanisms (e.g. soluble BCMA leading to off-target binding).6
Emerging data suggest that pretreatment with bispecific antibodies (BsAbs) or ADCs may reduce response rates and progression-free survival in patients receiving subsequent CAR T-cell therapy compared with BCMA-naïve populations.7–10 However, pretreatment with CAR T-cell therapy and ADCs appears to have a modest impact on efficacy in patients receiving subsequent BsAb therapy.11,12
CAR T-cell therapies and BsAbs provide targeted immuno-cytotoxicity but are associated with immune-mediated toxicities and infections, as well as higher care complexity.1,2
ADCs offer off-the-shelf outpatient therapy but are associated with ocular toxicities and require continuous administration.1,2
The optimal strategy to manage toxicity related to BCMA-targeted therapies involves tailoring dose and timing, balancing efficacy with tolerability, and an individualized treatment approach.12–15
Assuming equal access, CAR T-cell therapy is preferred over BsAb when both are options, and T-cell redirecting therapy (CAR-T/BsAb) is preferred ahead of BCMA-directed ADC, according to key recommendations from the International Myeloma Working Group and European Hematology Association–European Myeloma Network guidelines. However, recommendations are continually evolving with the publication of novel clinical and real-world data.16,17
Sequencing considerations for BCMA-directed therapies should take into account accessibility and logistical requirements, patient fitness, comorbidities, willingness to tolerate adverse events, timing of therapy, combination or sequential strategies, toxicity profile, persevering T-cell fitness and BCMA expression, patient preferences and goals of therapy, prior treatment, and BCMA exposure.
This independent educational activity was supported by GSK. All content was developed independently by SES in collaboration with the faculty. The funder was allowed no influence on the content of this activity.
References

