All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Symposium | Future directions with novel and established BCMA-DT in the multiple myeloma treatment paradigm
Featured:
Do you know... According to an international survey of >2,000 respondents, which of the following was highlighted as a key treatment goal for BOTH patients and HCPs?
On December 17, 2025, the Multiple Myeloma Hub held a virtual symposium, titled Integrating novel B-cell maturation antigen (BCMA)-directed therapies into clinical practice: Insights from real-world experience. During the symposium, Maria Victoria Mateos, University of Salamanca, Salamanca, ES, delivered a presentation covering future directions with novel and established BCMA-directed therapies in the multiple myeloma (MM) treatment paradigm.
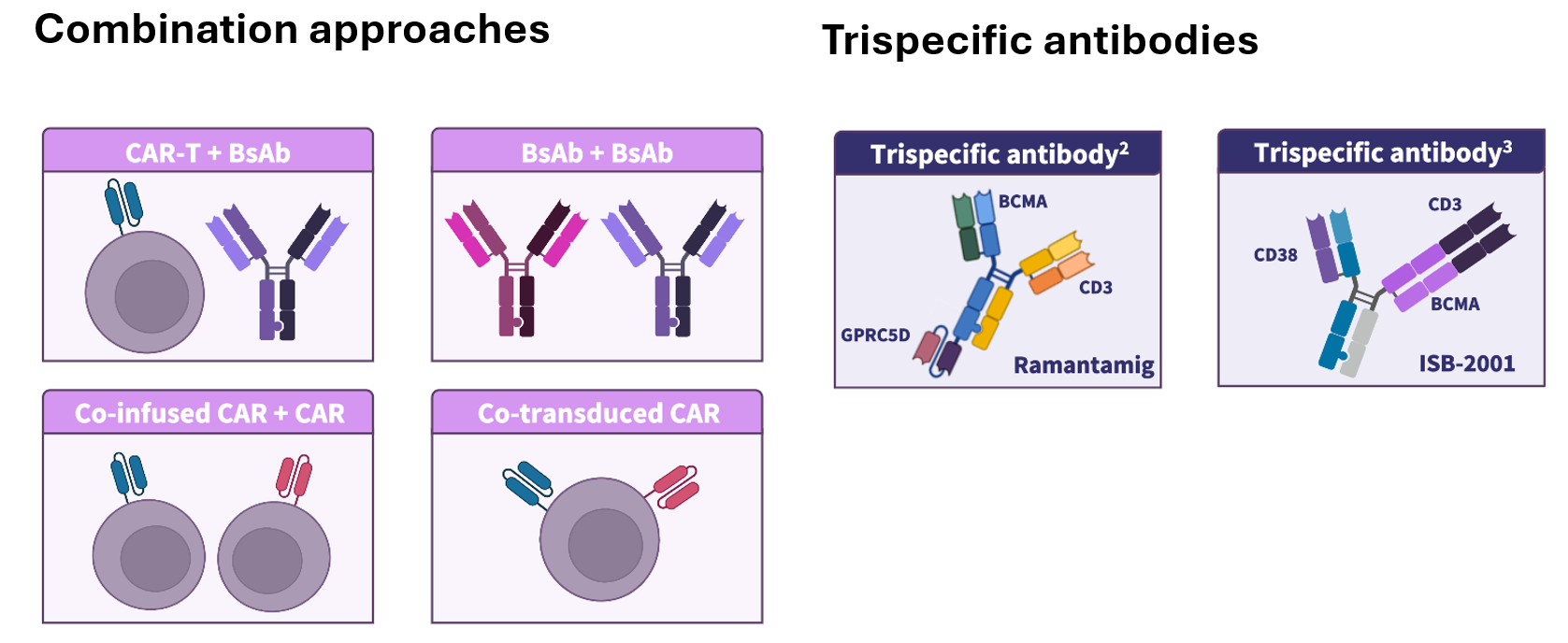
In this presentation, Mateos discussed the impact of recent BCMA-directed therapy approvals and indication changes on the treatment paradigm for relapsed/refractory MM (RRMM) (Figure 1), as well as the earlier incorporation of BCMA-directed therapies in MM treatment and their potential for use in newly diagnosed MM (NDMM) (Figure 1). Optimization of dosing schedules, treatment delivery, and community integration of BCMA-directed therapies were highlighted. The importance of multidisciplinary toxicity management, as well as shared decision-making to align treatment plans with patient goals, was also reviewed. Finally, future perspectives with BCMA-directed therapies, including novel treatment strategies, were shared (Figure 2).
Figure 1. Recent approvals and indication changes in RRMM*
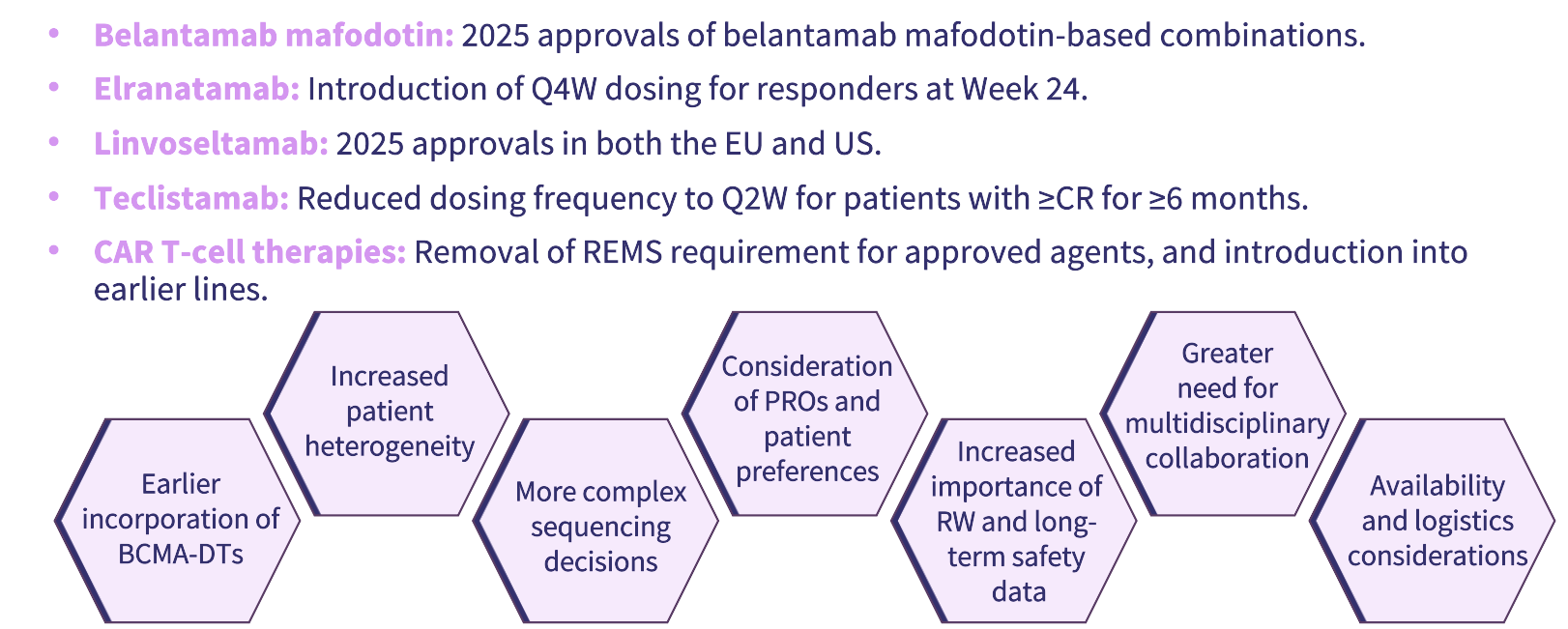
Figure 2. Future perspectives with BCMA-directed therapies*
Symposium | Future directions with novel and established BCMA-DT in the MM treatment paradigm
Key points
Recent approvals of BCMA-targeted treatments (chimeric antigen receptor [CAR] T-cell therapy, bispecific antibodies [BsAbs], and an antibody–drug conjugate [ADC]) have resulted in their rapid integration into clinical practice, increasing therapeutic options for RRMM while making sequencing decisions more complex.1
Real‑world and clinical data from the phase III DREAMM-7 and -8 studies support flexible dosing intervals for belantamab mafodotin-containing regimens (e.g. from every 3–4 weeks to 8–12 weeks) to reduce ocular toxicity; drug-specific dose optimization should be incorporated into clinical practice and trials.5
Successful implementation of BCMA-directed treatment regimens requires multidisciplinary teams (hematologists, nurses, neurologists, ophthalmologists, primary care physicians, infectious disease specialists, and pharmacists), standardized pathways for monitoring adverse events, including ocular events, and long‑term safety surveillance.6,7
Clinicians should integrate patient‑reported outcomes, treatment burden, logistics, and individual preferences into treatment selection and sequencing decisions to maximize adherence, quality of life, and real‑world benefit.1,8
Numerous treatment combination strategies and novel dual-antigen targeting agents are being actively investigated for the treatment of MM, with the potential to increase efficacy, reduce duration of response, and mitigate resistance mechanisms.2–4
Early-phase clinical trials evaluating CAR approaches that program patients’ T cells directly could eliminate leukapheresis, ex‑vivo manufacturing, and possibly lymphodepletion, offering faster, more scalable, and potentially lower‑cost CAR T-cell options.9
BCMA-directed therapies continue to evolve, with ongoing research refining how they are used across different stages of MM; shared decision-making remains central to treatment planning to ensure that therapy choices reflect both clinical considerations and individual patient preferences.
This independent educational activity was supported by GSK. All content was developed independently by SES in collaboration with the faculty. The funder was allowed no influence on the content of this activity.
References


 María-Victoria Mateos
María-Victoria Mateos


