All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Symposium | BCMA-directed therapies in MM: Current applications and the evolving clinical landscape
Featured:
Do you know... For patients with relapsed/refractory MM who are ineligible for or unable to access T-cell-redirecting therapies, which of the following BCMA-directed therapies would you recommend?
On December 17, 2025, the Multiple Myeloma Hub held a virtual symposium, titled Integrating novel B-cell maturation antigen (BCMA)-directed therapies into clinical practice: Insights from real-world experience. During the symposium, Marc-Andrea Bärtsch, Heidelberg University Hospital, DE, delivered a presentation covering the current applications of BCMA-directed therapies in multiple myeloma (MM) and the evolving clinical landscape.
In this presentation, Bärtsch discussed the rationale for targeting BCMA in MM and reviewed pivotal clinical trial data supporting the approval of BCMA-directed therapies. Bärtsch outlined the mechanisms of action (Figure 1), clinical positioning, and key efficacy and safety findings for antibody–drug conjugates (ADCs), bispecific antibodies, and chimeric antigen receptor (CAR) T-cell therapies, highlighting differences in availability, toxicity profiles, and durability of response across treatment modalities and lines of therapy.
Symposium | BCMA-directed therapies in MM: Current applications and the evolving clinical landscape
Figure 1. Modalities of BCMA-directed therapies for MM*
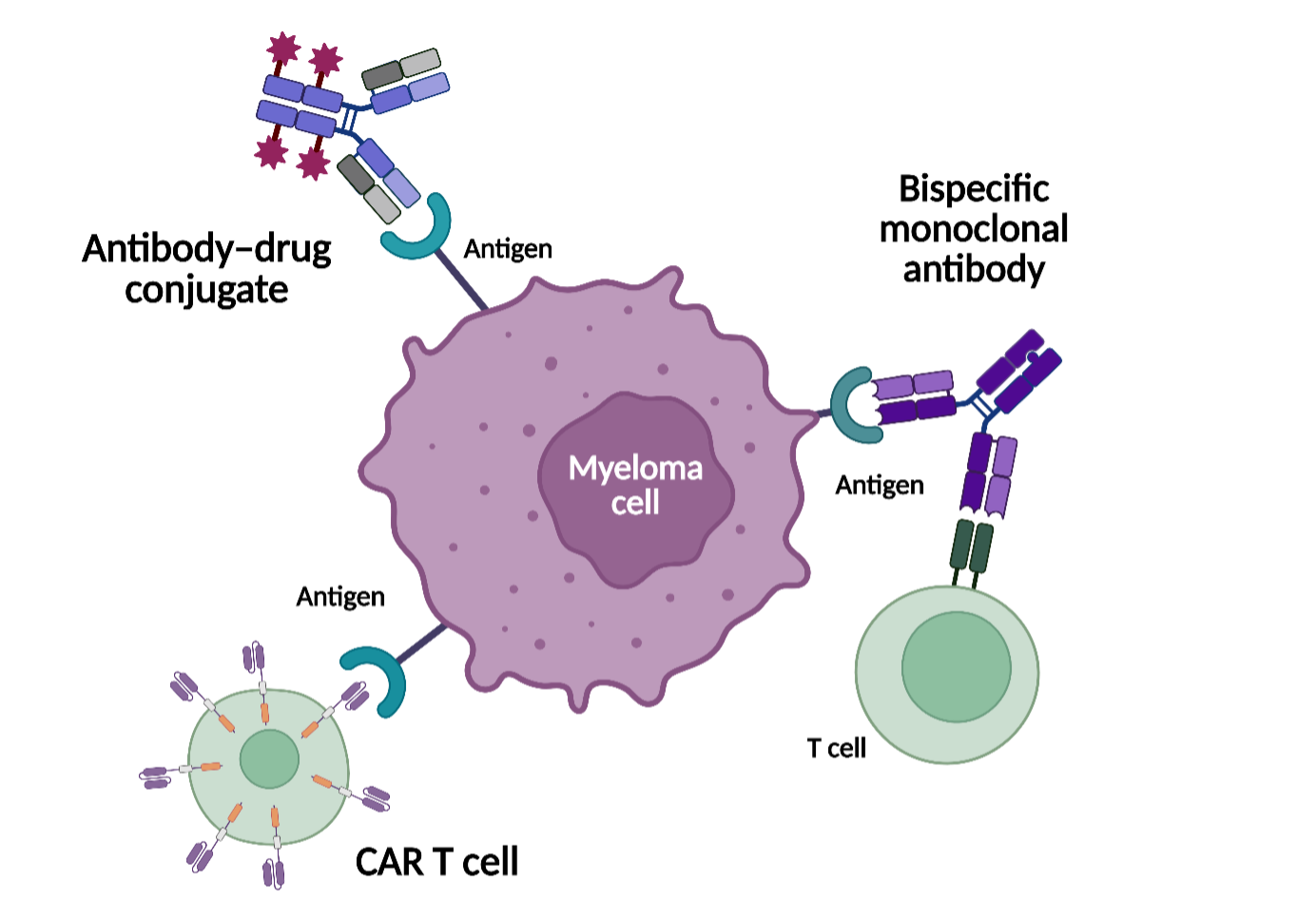
Key points
- BCMA is a key target in MM due to its role in plasma cell biology, generally stable expression across disease stages in BCMA-naïve patients, and limited expression outside the plasma cell lineage.2–4
- Currently approved BCMA-directed treatment approaches include ADCs, which deliver a cytotoxic payload to BCMA-expressing myeloma cells; bispecific antibodies, which engage T cells to mediate tumor cell killing; and CAR T-cell therapies, which use genetically modified autologous T cells targeting BCMA.1
- ADCs do not rely on T-cell engagement and may therefore be considered as a treatment option for selected patients who are ineligible for or unable to access T-cell-redirecting therapies.1
- ADC-based regimens, including belantamab mafodotin-based combinations, have demonstrated clinically meaningful activity in early relapsed MM. Ocular toxicities are generally manageable but often require dose modification, treatment interruption, and ophthalmologic monitoring.5,6
- BCMA-directed bispecific antibodies have demonstrated activity in heavily pretreated patient populations, with generally low rates of high-grade cytokine release syndrome and neurotoxicity. However, treatment is associated with an increased risk of infections, necessitating close monitoring and supportive care measures, including prophylaxis.7
- BCMA-targeted CAR T-cell therapies can achieve deep and durable responses, including sustained measurable residual disease negativity, particularly when administered in earlier lines of therapy. Use of these therapies also requires consideration of logistics, manufacturing time, and the need for specialized centers and toxicity management.8
- Differences in mechanisms of action and resistance among BCMA-directed therapies support their potential sequential use. Antigen loss, mutation, or downregulation may limit long-term efficacy and highlight the need for continued optimization of treatment strategies.
This independent educational activity was supported by GSK. All content was developed independently by SES in collaboration with the faculty. The funder was allowed no influence on the content of this activity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

