All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Selinexor, pomalidomide, and dexamethasone for the treatment of relapsed/refractory multiple myeloma
Selinexor is an oral first-in-class selective inhibitor of nuclear export that inhibits exportin 1 (XPO1). XPO1 is responsible for the nuclear export and inactivation of tumor suppressor proteins and is overexpressed in patients with multiple myeloma (MM). Currently, selinexor has received conditional marketing authorization from the European Commission, in combination with dexamethasone, and has been approved by the U.S. Food and Drug Administration (FDA) in combination with bortezomib and dexamethasone.
The selinexor and backbone treatment of multiple myeloma patients (STOMP) trial has been setup to assess 10 different treatment combinations with selinexor, in 11 arms in patients with newly diagnosed and refractory MM. Combinations that appear promising will move forward to separate phase II/III studies. A previous report of selinexor combined with daratumumab and dexamethasone, or with carfilzomib and dexamethasone can be found here.
During the 7th World Congress on Controversies in Multiple Myeloma (COMy21), Christine Chen reported on the results of the selinexor + pomalidomide + dexamethasone (SPd) arm in the phase Ib/II STOMP trial (NCT02343042)1, and an update was presented at this year’s virtual 2021 ASCO Annual Meeting by Darrell White.2
Study design
Selinexor was tested at 60, 80, or 100 mg once a week, or 60 or 80 mg twice a week with pomalidomide and dexamethasone. The current recommended dose for selinexor is 80 mg in combination with 20 mg dexamethasone on Days 1 and 3 of each week.3
Primary endpoints:
- Maximum tolerated dose.
- Recommended phase 2 dose (RP2D).
- Overall response rate (ORR).
Secondary endpoints:
- Safety and tolerability per NCI Common Terminology Criteria for Adverse Events.
- Progression-free survival (PFS).
- Overall survival (OS).
Eligibility criteria:
- Refractory to, or progressing on, a previous regimen.
- Undergone ≥ 2 cycles of lenalidomide and a proteosome inhibitor previously.
- Prior pomalidomide is allowed, but pomalidomide-refractory patients are only allowed in the dose escalation phase.
- Patients with smoldering MM, non-secretory MM, or active plasma cell leukemia were excluded.
Results
Patient characteristics
A total of 72 patients were included in the trial (50% women) with an average age of 64.0 years. The majority of patients were ISS Stage I or II and had received a median of four lines of therapy previously. Most patients had been treated with lenalidomide (100%) and bortezomib (91.7%) and were refractory to them (80.6% and 50%, respectively). The RP2D was received by 20 patients, of whom 70% had been given a stem cell transplant compared to 80.6% in the whole cohort (Table 1).
Table 1. Baseline characteristics*
|
ISS, International Scoring System; mAb, monoclonal antibody; RP2D, recommended phase 2 dose; XPd, selinexor, pomalidomide, and dexamethasone. |
||
|
Characteristic |
Total (N = 72) |
RP2D (N = 20) |
|---|---|---|
|
Median age, years (range) |
64.0 (37−85) |
65.5 (37−85) |
|
Females, % |
50 |
65 |
|
Median time from diagnosis to XPd treatment, years (range) |
4.4 (0.9−22.8) |
3.4 (1.1−9.2) |
|
ISS Stage, % |
||
|
I |
30.6 |
35.0 |
|
II |
25.0 |
15.0 |
|
III |
13.9 |
15.0 |
|
Missing |
30.6 |
35.0 |
|
Median number of prior regimens (range) |
4 (1−12) |
4 (1−12) |
|
Lenalidomide, % |
||
|
Treated/refractory |
100.0/ 80.6 |
100.0/ 80.0 |
|
Pomalidomide, % |
||
|
Treated/refractory |
29.2/ 26.4 |
20.0/ 15.0 |
|
Bortezomib, % |
||
|
Treated/refractory |
91.7/ 50.0 |
85.0/ 45.0 |
|
Carfilzomib, % |
||
|
Treated/refractory |
43.1/ 37.5 |
60.0/ 50.0 |
|
Anti-CD38 mAb, % |
||
|
Treated/refractory |
30.6/ 27.8 |
30.0/ 25.0 |
|
Stem cell transplant, % |
80.6 |
70.0 |
Safety
The majority of dose-limiting toxicities (DLTs) were experienced at 80 mg selinexor with all doses of pomalidomide (2, 3, and 4 mg). DLTs included Grade III febrile neutropenia, Grade II/IV neutropenia, Grade III/IV thrombocytopenia, and one Grade V pneumonia.
From these results, the determined RP2D is shown in Figure 1.
Figure 1. Recommended phase II dosing schedule*
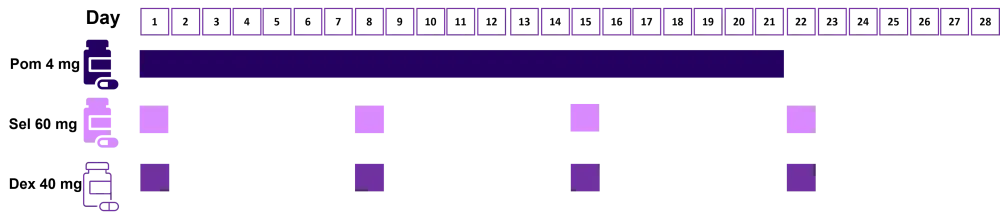
Dex, dexamethasone; Pom, pomalidomide; Sel, selinexor.
*Adapted from Chen et al.1
The most common treatment-related adverse events (TRAEs) were neutropenia, nausea, anemia, and fatigue. The only Grade IV TRAEs were hematologic (Table 2). No unexpected TRAEs were identified for this treatment combination and TRAEs were managed with supportive care and/or dose modifications.
Table 2. TRAEs*
|
RP2D, recommended phase 2 dose; TRAE, treatment-related adverse event. |
||||||
|
TRAE |
TRAEs (≥20% patients) |
RP2D |
||||
|---|---|---|---|---|---|---|
|
Any grade |
Grade III |
Grade IV |
Any grade |
Grade III |
Grade IV |
|
|
Hematologic |
||||||
|
Neutropenia† |
61.1 |
26.4 |
26.4 |
75.0 |
35.0 |
25.0 |
|
Anemia |
52.8 |
29.2 |
0 |
65.0 |
25.0 |
0 |
|
Thrombocytopenia‡ |
48.6 |
15.3 |
12.5 |
45.0 |
15.0 |
10.0 |
|
Leukopenia |
25.0 |
8.3 |
5.6 |
25.0 |
5.0 |
0 |
|
Gastrointestinal |
||||||
|
Nausea |
61.1 |
1.4 |
0 |
70.0 |
0 |
0 |
|
Decreased appetite |
41.7 |
1.4 |
0 |
30.0 |
0 |
0 |
|
Diarrhea |
29.2 |
0 |
0 |
25.0 |
0 |
0 |
|
Vomiting |
22.2 |
1.4 |
0 |
20.0 |
0 |
0 |
|
Constitutional |
||||||
|
Fatigue |
54.2 |
9.7 |
0 |
65.0 |
5.0 |
0 |
|
Weight decrease |
36.1 |
0 |
0 |
25.0 |
0 |
0 |
Efficacy
For all patients in the trial, a median PFS of 10.4 months (95% CI, 8.7−not estimable) was observed, with a median duration of response (DOR) of 10.3 months (95% CI, 7.9−not estimable). In the RP2D group, the median PFS, DOR, and OS were not reached. Interestingly, in the 16 pomalidomide-refractory patients, the median PFS was 8.7 months with a median OS of 8.0 months. For the 19 patients who had received an anti-CD38 monoclonal antibody, median PFS was also 8.7 months, while the median OS increased to 9.6 months. However, pomalidomide-naïve patients showed the longest PFS at 12.2 months (n = 50) with a DOR of 24.2 months.
In the RP2D group, ORR was 65% with 5% of patients achieving a stringent CR (sCR). In the pomalidomide-refractory group, the ORR was only 43.8% with no patients achieving CR (Table 3).
Table 3. Best responses in evaluable patients*
|
CR, complete response; mAb, monoclonal antibody; MR, minimal response; ORR, overall response rate; PD, disease progression; Pom, pomalidomide; PR, partial response; QW, weekly; RP2D, recommended phase 2 dose; sCR, stringent CR; SD, stable disease; Sel, selinexor; VGPR, very good partial response. |
|||||
|
Evaluable patients |
ORR |
sCR + CR |
VGPR |
PR |
MR + SD |
|---|---|---|---|---|---|
|
RP2D: 60 mg Sel (QW) + 4 mg Pom |
65.0 |
5.0 |
25.0 |
35.0 |
25.0 |
|
Pom-naïve or nonrefractory |
56.8 |
4.6 |
18.2 |
34.1 |
36.3 |
|
Pom-refractory |
43.8 |
0.0 |
12.5 |
31.3 |
56.3 |
|
Pretreated with anti-CD38 mAb |
57.9 |
5.3 |
15.8 |
36.8 |
42.1 |
Conclusion
The combination of SPd did not result in any unexpected TRAEs and could be managed with supportive care. SPd treatment resulted in an ORR of 65% at the RP2D. A median PFS of 12.2 months was achieved in patients who were nonrefractory or naïve to pomalidomide. These results support the assessment of SPd in a phase III study, where this combination will be compared against elotuzumab + Pd in relapsed/refractory patients with MM who have been previously treated with a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

