All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Selinexor triplets for relapsed/refractory multiple myeloma—an alternative to IMiD-based therapy?
The nuclear export protein, exportin 1 (XPO1), is overexpressed in multiple myeloma (MM), resulting in a decreased abundance of tumor suppressor proteins in the nucleus. Selinexor is a first-in-class, orally available, selective inhibitor of nuclear export (SINE), which binds to and inactivates XPO1, reinstating physiological tumor suppressor levels.1
Data from part two of the STORM study provided grounds for the U.S. Food and Drug Administration (FDA) approval of selinexor in combination with low dose dexamethasone (dex) for the treatment of patients with relapsed/refractory MM (RRMM). Here, we provide a summary of the latest advances of selinexor in triplet regimens for the treatment of RRMM, as presented at this year’s virtual American Society of Clinical Oncology (ASCO) Annual Meeting.
STOMP1,2
The STOMP study (NCT02343042) is a phase Ib/ II dose escalation and expansion trial evaluating selinexor in combination with eight established MM backbone therapies across nine arms for the treatment of patients with RRMM. During this year’s ASCO Meeting, Cristina Gasparetto discussed the data from two arms of the STOMP study—watch the interview here. The Multiple Myeloma Hub is pleased to present a summary.
- STOMP primary endpoints: Maximum tolerated dose and recommended phase II dose (RP2D) of respective treatment regimen
- STOMP secondary endpoints: Overall response rate (ORR), progression-free survival (PFS), and duration of response
Selinexor, daratumumab, and dexamethasone (SDd; #8510)1
Selinexor and daratumumab (dara) have both demonstrated single-agent clinical activity in MM. One arm of the STOMP study sought to determine the efficacy and safety profiles of SDd in patients with RRMM.
Study design
- The study enrolled adult patients with MM who
- had received ≥ 3 lines of prior therapy, including an immunomodulatory drug (IMiD®) and a proteasome inhibitor (PI), or
- were refractory to an IMiD and a PI
- Patients in the dose-expansion cohort were anti-CD38 monoclonal antibody naïve
- The dose escalation phase followed a 3 × 3 design and enrolled patients in two cohorts to receive
- 60 mg selinexor biweekly (BIW) + 16 mg/kg dara weekly (QW; n = 3) or
- 100 mg selinexor QW + 16 mg/kg dara QW (n = 6)
Results
- Dose-limiting toxicities were observed in two of the patients receiving BIW selinexor, but none were reported in the QW cohort
- RP2D: QW selinexor 100 mg + dara 16 mg/kg + dex 40 mg
- As of January 2, 2020, 34 patients had been enrolled onto the SDd arm of the STOMP study (Table 1)
Table 1. Baseline characteristics of patients enrolled on the STOMP SDd arm1
|
Auto-SCT, autologous stem cell transplantation; BIW, biweekly; dara, daratumumab; IMiD, immunomodulatory drug; PI, proteasome inhibitor; QW, weekly; SDd, selinexor + daratumumab + dexamethasone * RP2D. † Patients pre-treated with bortezomib, carfilzomib, lenalidomide, and pomalidomide. |
|
|
Characteristic |
N = 34 |
|
Regimen, n |
|
|
BIW selinexor |
3 |
|
QW selinexor* |
31 |
|
Median age, years (range) |
68 (44–83) |
|
Male, % |
56 |
|
Median time from diagnosis to SDd, years (range) |
5.6 (<1–14) |
|
Median prior regimens, n (range) |
3 (2–10) |
|
Prior treatment, % |
|
|
PI (exposed:refractory) |
100:85 |
|
IMiD (exposed:refractory) |
100:76 |
|
Quad exposed† |
23.5 |
|
Auto-SCT |
73.5 |
|
Dara treated |
6 |
Efficacy
- Median PFS: 12.5 months across all groups
- Patient outcomes are presented in Figure 1
Figure 1. Patient outcomes to SDd treatment regimen1
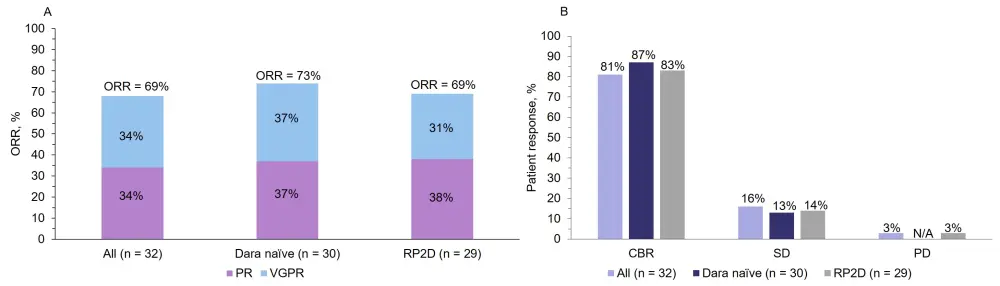
A ORRs and B survival outcomes to SDd in all enrolled patients, patients who are dara naïve, and patients who received the RP2D.
CBR, clinical benefit rate; dara, daratumumab; ORR, overall response rates; PD, progressive disease; PR, partial response; RP2D, recommended phase II dose; SD, stable disease; SDd, selinexor + daratumumab + dexamethasone; VGPR, very good partial response
CBR = ORR + minimal response
Safety
- No deaths due to treatment-related adverse events (TRAEs) or serious AEs (SAEs) were reported
- Common Grade 3–4 TRAEs are outlined in Table 2
Table 2. Common Grade 3–4 TRAEs observed in > 5% of patients receiving SDd at the RP2D1
|
RP2D, recommended phase II dose; TRAE, treatment-related adverse event |
|
|
Grade 3–4 TRAEs |
% of patients (N = 34) |
|
Thrombocytopenia |
47.0 |
|
Anemia |
32.4 |
|
Neutropenia |
26.5 |
|
Fatigue |
17.6 |
|
Hyponatremia |
11.8 |
|
Nausea |
8.8 |
Study conclusions
The study identified an SDd treatment regimen that was well tolerated in patients with PI- and IMiD-refractory MM and suggests that clinical improvement is achievable with the RP2D.
Selinexor + carfilzomib + dexamethasone (SKd; #8530)2
This study set out to determine the efficacy and safety profiles of SKd in patients with RRMM.
Study design
- Adult patients with MM progressing or refractory to a previous regimen, excluding carfilzomib, were enrolled
- The dose escalation phase followed a 3 × 3 design; the SKd dosing schedules are shown in Table 3
Table 3. Dosing schedules of SKd2
|
IV, intravenous; PO, per os * C1D1 dose of carfilzomib was 20 mg/m2 for all patients |
|||
|
Dose level |
Selinexor, mg PO Days 1, 8, 15, 22 |
Dex, mg IV or PO Days 1, 8, 15, 22 |
Carfilzomib, mg/m2 IV* Days 1, 8, 15 |
|
1 |
100 |
40 |
56 |
|
–1 |
80 |
40 |
56 |
|
–1a |
80 |
40 |
70 |
Results
- Dose-limiting toxicities were observed in two patients in both the 1 and –1a dosing cohorts, but none were reported in the –1 cohort
- RP2D: QW selinexor 80 mg + carfilzomib 56 mg/m2 + dex 40 mg
- As of May 1, 2020, 24 patients had been enrolled onto the SKd arm of the STOMP study (Table 4)
Table 4. Baseline characteristics of patients enrolled on the STOMP SKd arm2
|
SCT, stem cell transplantation; SKd, selinexor + carfilzomib + dexamethasone *n = 23 |
|
|
Characteristic |
N = 24 |
|
Median age, years (range) |
70.5 (50–76) |
|
Male, % |
62.5 |
|
Median time from diagnosis to SKd, years (range)* |
5.01 (2.7–11.3) |
|
Median prior regimens, n (range)* |
3 (1–8) |
|
Prior treatment, % |
|
|
Bortezomib |
100 |
|
Carfilzomib |
4.2 |
|
Lenalidomide |
95.8 |
|
Pomalidomide |
62.5 |
|
Daratumumab |
58.3 |
|
SCT |
79.2 |
Efficacy
- Median PFS: Not reached
- Patient outcomes are presented in Figure 2
Figure 2. Patient outcomes to SKd treatment regimen2
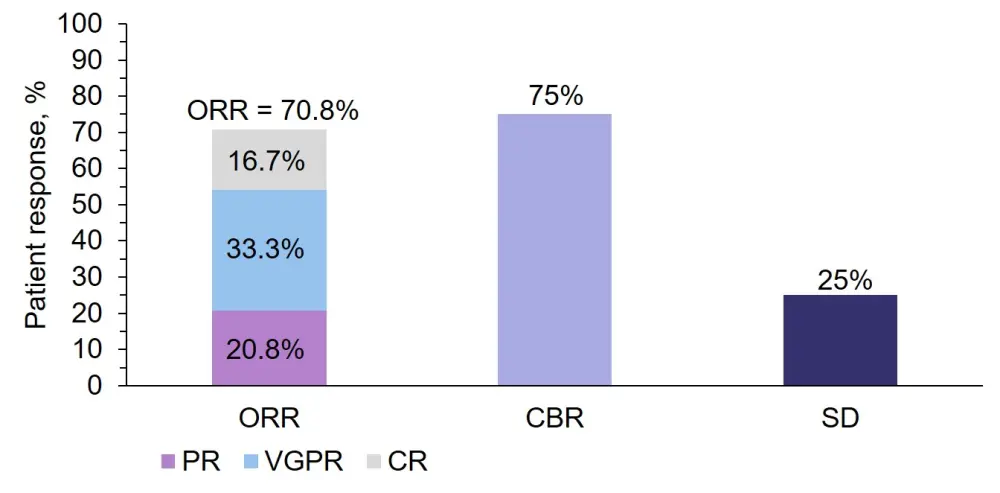
CBR, clinical benefit rate; CR, complete response; PR, partial response; SD, stable disease; VGPR, very good partial response
CBR = ORR + minimal response.
Safety
- No deaths due to TRAEs or serious AEs were reported
- Common Grade 3–4 TRAEs are outlined in Table 5
Table 5. Common Grade 3–4 TRAEs observed in > 5% of patients receiving SKd at the RP2D2
|
RP2D, recommended phase II dose; TRAE, treatment-related adverse event *Data cutoff: May 1, 2020 |
|
|
Grade ≥ 3 TRAEs |
% of patients (N = 24)* |
|
Thrombocytopenia |
54 |
|
Anemia |
20.8 |
|
Leukopenia |
12.5 |
|
Neutropenia |
8.3 |
|
Fatigue |
8.3 |
Study conclusions
QW SKd demonstrated deep responses and appears to be well tolerated in patients with RRMM following a median of four prior lines of therapy. These data support further investigation of the regimen.
BOSTON study (#8501)3
The phase III BOSTON trial (NCT03110562) aimed to compare the efficacy of selinexor + bortezomib + dex (SVd) with bortezomib + dex (Vd), and to determine if SVd reduces the rate of PN compared with Vd alone. At this year’s virtual ASCO Annual Meeting, Meletios A. Dimopoulos outlined the initial results, summarized here.3
Conclusion
Data from the aforementioned studies support the incorporation of selinexor into triplet regimens for the treatment of MM. Selinexor appears to enhance the clinical efficacy of currently established regimens and could provide an alternative to IMiD-based therapeutics in the relapsed setting.
Additional resources
For more information on the current status of selinexor for the treatment of R/R diffuse large B-cell lymphoma, click here.
Expert Opinion
 Cristina Gasparetto
Cristina GasparettoReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

