All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Do you know... A 75-year-old woman with RRMM presents with a steadily progressing disease after a 4th line of therapy. She has received two PIs, two IMiDs, and one anti-CD38 antibody. She is also being treated for hypertension and diabetes, but her general performance for continued treatment is favorable. In an ideal scenario where all approved products are available, what is the optimal choice as the following line of therapy?
Video series
During the European Hematology Association (EHA) 2023 Hybrid Congress, the Lymphoma Hub and Multiple Myeloma Hub held a joint satellite session on the ins and outs of CAR T-cells in the real world.
Here the Multiple Myeloma Hub is pleased to share a real-world patient case as presented by Doris Hansen, Moffitt Cancer Center, Tampa, US.
Real-world patient cases: CAR T-cell therapy
Here, Hansen discusses a case study of a 78-year-old caucasian male with multiple comorbidities and prior exposure to a BCMA-directed agent (Figure 1), they were treated with idecabtagene vicleucel (ide-cel). Hansen uses the case study to examine real-world safety and efficacy data for both ide-cel (Figure 2) and ciltacabtagene autoleucel (cilta-cel), making comparisons with clinical trial data from KarMMa and CARTITUDE-1.1
Figure 1. Patient case study outline*

BCMA, B-cell maturation antigen; CAD/NSTEMI, coronary artery disease/non-ST-elevation myocardial infarction; CAR, chimeric antigen receptor; cyclo; cyclophosphamide; dara, daratumumab; d, dexamethasone; ECOG, Eastern Cooperative Oncology Group; EF, ejection fraction; HCT, hematopoietic stem cell transplant; IDDM, insulin-dependent diabetes mellitus 2; ide-cel, idecabtagene vicleucel; Ig, immunoglobulin; Isa, isatuximab; Ixa, ixazomib; K, carfilzomib; MM, multiple myeloma; P, pomalidomide; R, lenalidomide; R-ISS, revised International Staging System; s/p, status post; T, thalidomide; V, bortezomib
*Provided by Hansen.1
Figure 2. Safety of ide-cel in the real-world setting*
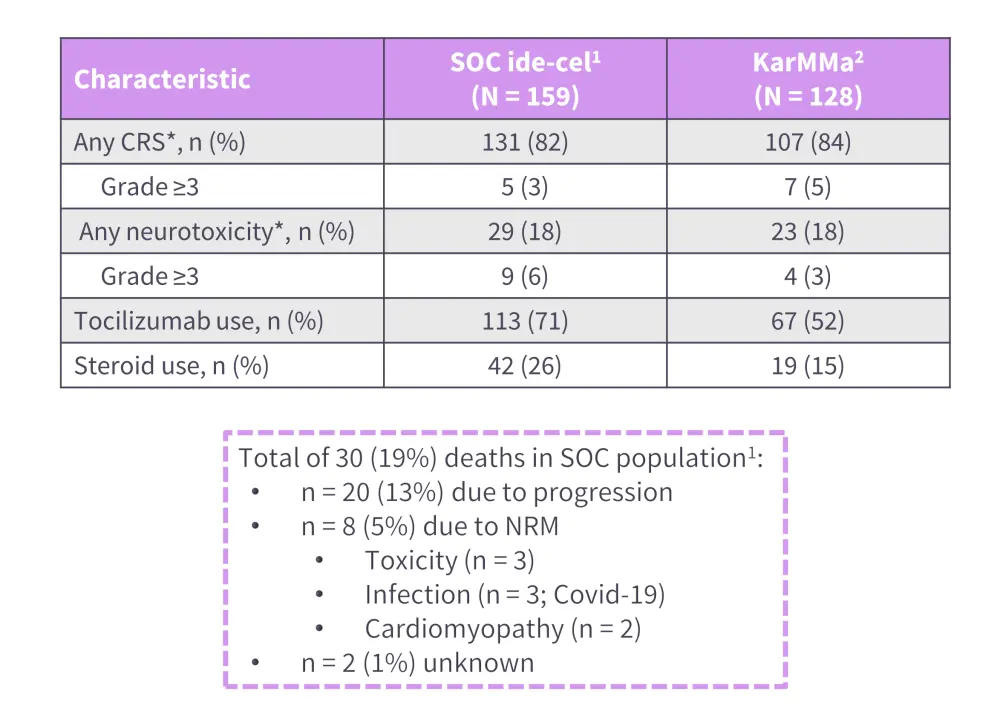
CRS, cytokine release syndrome; ide-cel, idecabtagene vicleucel; NRM, non-relapse mortality; NT, neurotoxicity; SOC, standard of care.
*Adapted from Hansen, et al.2
†ASTCT criteria used for grading CRS and NT.
Watch or download the presentation to learn more about:
- The safety and efficacy of ide-cel and cilta-cel in the real world compared with clinical trial data
- The factors associated with poorer response to CAR T cells
- The discrepancy between real-world patient characteristics and eligibility criteria for clinical trials of CAR T cells
- The use of CAR therapies in patients ineligible for clinical trials
Key points1,2
- Best overall response rate to ide-cel and cilta-cel in standard of care setting, 84% and 89%, respectively.
- Factors associated with poorer response to ide-cel include,
- prior use of BCMA-targeted therapies;
- high-risk cytogenetics;
- ECOG performance status ≥2; and
- younger age.
- High-risk cytogenetics were an independent predictor of inferior outcomes with cilta-cel use.
- Overall, ≥50% of real-world patients with multiple myeloma are ineligible for enrollment in clinical trials of CAR T cells but demonstrated favorable safety and efficacy data in the standard-of-care setting, compared with KarMMa and CARTITUDE-1.
- Treatment with CAR T-cell therapies such as ide-cel and cilta-cel is feasible in the real-world setting, including in patients ineligible for clinical trial
This activity was supported through an educational grant from Bristol Myers Squibb.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Doris Hansen
Doris Hansen

