All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Do you know... Which of the following features have NOT been associated with shortened duration of response with CAR T-cell therapy in multiple myeloma?
Video series
During the European Hematology Association (EHA) 2023 Hybrid Congress, the Lymphoma Hub and Multiple Myeloma Hub held a joint satellite session on the ins and outs of CAR T-cells in the real world.
Here the Multiple Myeloma Hub is pleased to share the presentation by Shaji Kumar, Mayo Clinic, Rochester, US, on biomarkers and eligibility for CAR T-cell therapies in multiple myeloma.
In this presentation, Shaji Kumar shares the currently approved CAR T-cell products for MM, discussing their up-to-date clinical data (Figure 1). He also shares the limitations and logistical challenges facing the implementation CAR T-cell therapy, as well as the current eligibility criteria for its use (Figure 2).
Figure 1. Efficacy and safety data for KarMMa and CARITITUDE-1*
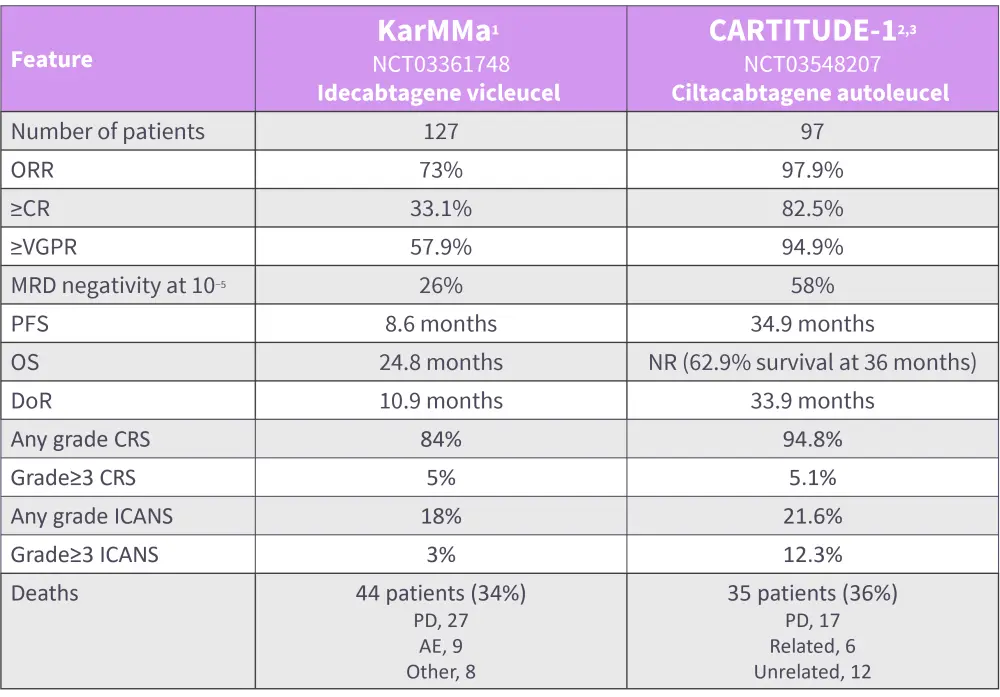
AE, adverse event; CR, complete response; CRS, cytokine release syndrome; DoR, duration of response; ICANS, mmune effector cell-associated neurotoxicity syndrome; MRD, minimal residual disease; NR, not reached; ORR, overall response rate; PD, progressive disease; PFS, progression-free survival; OS, overall survival; VGPR, very good partial response.
*Adapted Chekol Abebe, et al.,1 Martin T, et al.,2 and Munshi, et al.3
Figure 2. Eligibility criteria for the use of CAR T-cell products*
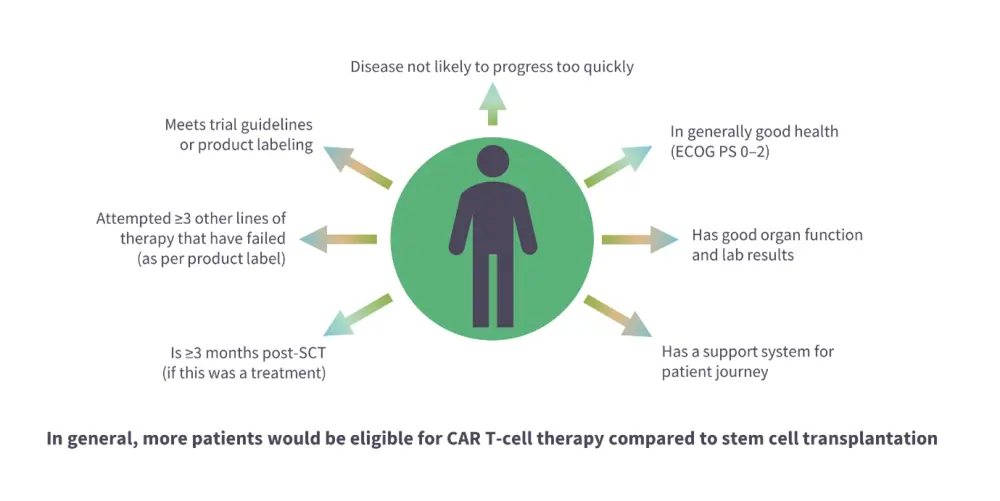
CAR, chimeric antigen receptor; ECOG PS, Eastern Cooperative Oncology Group Performance Status; SCT, stem cell transplant.
*Adapted from Dave, et al.4
Watch or download the presentation to learn more about:
- The myeloma treatment paradigm
- The currently approved CAR T-cell products in multiple myeloma, including their up-to-date safety and efficacy data
- The impact of prior treatment with BCMA therapy on clinical outcomes with CAR T-cell therapy
- The challenges and logistics when considering CAR-T cell therapy
- The limitations of CAR T-cell products
- Eligibility criteria for CAR T-cell therapy
Key points
- Currently, there are no individual biomarkers to definitively determine eligibility for treatment with CAR T-cell therapy.
- Idecabtagene vicleucel and ciltacabtagene autoleucel are approved for use in triple class exposed RRMM; however, these patients
- are more likely to be of an advanced age and possess other comorbidities that may result in increased toxicity;
- are more likely to have been exposed to a BCMA-targeted therapy, which has been found to result in lower response rates;
- have typically faster rates of disease progression, meaning that CAR T-cells may not be produced in time; and
- have a high tumor burden and therefore increased risk of cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome.
- Research is currently on the feasibility of CAR T-cell therapy in earlier lines of therapy is ongoing.
- Limited access to therapies as well as the costs associated with treatment are logistical factors when considering CAR T-cell therapy.
This activity was supported through an educational grant from Bristol Myers Squibb.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Shaji Kumar
Shaji Kumar

