All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
GPRC5D-directed CAR T-cell therapy and bispecific antibodies for patients with RRMM: Latest updates from ASH 2022
Although B-cell maturation antigen (BCMA)-directed immunotherapies have induced deep responses and prolonged survival in patients with multiple myeloma (MM), the majority will still experience disease progression.1 Therefore there is a need for more potent and differentiated therapeutic options for these patients.1
The Multiple Myeloma Hub has previously highlighted the rationale of targeting G protein-coupled receptor family C group 5 member D (GPRC5D) in MM, including its higher and differentiated expression in MM cells compared with healthy tissue and its expression independent of BCMA expression.1,2
In this article, we summarize the latest data regarding specific GPRC5D-targeted CAR T-cell therapy and bispecific antibodies for patients with relapsed/refractory (R/R) MM, including recent data presented during the 64th American Society of Hematology (ASH) Annual Meeting and Exposition.
GPRC5D-directed bispecific antibodies
Talquetamab is an anti-GPRC5D bispecific antibody that has received breakthrough therapy designation from the U.S. Food and Drug Administration (FDA) for patients with R/R MM based on the latest data from the MonumenTAL-1 trial (NCT03399799) reported here.
Forimtamig is another novel bispecific GPRC5D T-cell-engaging bispecific antibody. Its dual binding 2:1 mode of action and biomarker analysis from the first-in-human clinical trial were previously reported on the Multiple Myeloma Hub. Here, we report on the following:
- Updated intravenous (IV) data and the initial subcutaneous (SC) data from forimtamig (also known as RG6234) presented by Carlo-Stella2
- Updated biomarker analysis for SC and IV arms presented by Broeske3
- Preclinical activity results on forimtamig compared with a 1:1 GPRC5D-T-cell bispecific (talquetamab) and a 2:1 BCMA-TCB (alnuctamab) presented by Eckmann1
Updated IV and first SC data of forimtamig in R/R MM2
- Overall, 51 patients were allocated to the IV arm and 57 patients to the SC arm
- At baseline, 50% of patients presented with high-risk cytogenetic features
- In the IV versus SC arms, respectively:
- The median number of prior lines of therapy was five and four
- 62% and 72% of patients were triple-class refractory
- 36% and 42% of patients were penta-class refractory
- 20% of patients received prior anti-BCMA therapy in both arms
Efficacy
At data cut-off (October 21, 2022), the overall response rate for evaluable patients across all tested doses in the IV and SC arms were 71.4% and 63.6%, respectively (Figure 1). Of note, 10 out of 14 patients across both arms achieved a minimal residual disease (MRD)-negative complete response (CR) at 10−5 in a next-generation sequencing-based analysis. The median duration of response was 10.8 months and 12.5 months for the IV and SC arms, respectively.
Figure 1. Response rates for IV (dose, 18–10,000 µg) and SC (dose, 30–72,000 µg) arms*
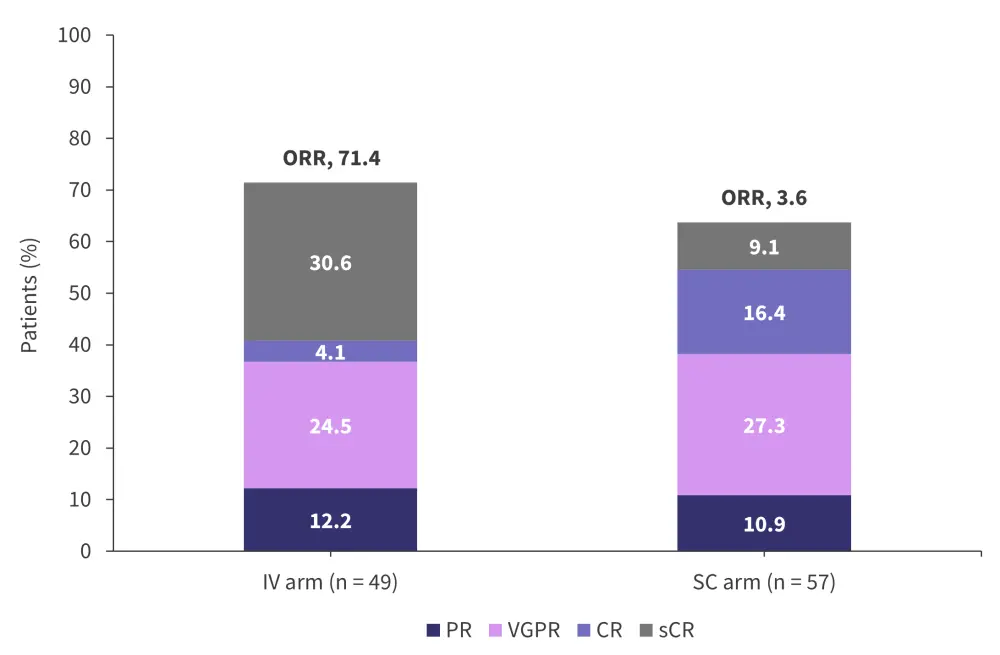
CR, complete response; IV, intravenous; ORR, overall response rate; PR, partial response; SC, subcutaneous; sCR, stringent CR; VGPR, very good partial response.
*Adapted from Carlo-Stella.2
Safety
Grade 3–4 adverse events (AEs) occurred in 68% and 74% of the IV and SC arms, respectively.
- Grade 5 AEs were reported in five patients across both arms, one related to forimtamig.
- Eight patients across both arms experienced AEs that led to dose reduction and discontinuation of forimtamig.
- Hematologic AEs reported in both arms included anemia, thrombocytopenia, and neutropenia.
- The most common non-hematologic AE reported for both arms was cytokine release syndrome (CRS), with 82.4% and 78.9% cases in the IV and SC arms, respectively.
- Most incidences of CRS were Grade 1 or 2 and occurred in Cycle 1, with one patient in each arm reporting a Grade ≥3 CRS (Grade 3, IV arm and Grade 4, SC arm); and the majority managed by corticosteroids and tocilizumab.
- Incidence of any-grade central nervous system AEs consistent with immune effector cell-associated neurotoxicity syndrome (ICANS) was roughly 10% in both arms; three patients experienced a Grade ≥3 of ICANS.
- Any-grade skin toxicities occurred in 78.4% of patients in the IV arm versus 86% in the SC arm. Mucosal and hair and nail changes were mostly Grade 1 or 2 and observed in Cycle 1.
- COVID-19 was a prominent infection, with 21–25% of any-grade cases reported across both arms.
Updated biomarker analysis of forimtamig in IV and SC arms3
Results from the biomarker analysis of forimtamig by administration route are as follows:
- Across all tested doses, both IV and SC administrations led to cytokine release.
- After the first dose, SC administration resulted in a delayed cytokine release.
-
- This peaked 24–72 hours after the first dose.
- This was a 1.7–23.7-fold lower cytokine release when compared with a 4–24-hour peak cytokine release in the IV arm.
- T-cell activation was observed in peripheral blood, indicated by CD8+ expression/margination 4 hours and 24 hours after IV and SC administration, respectively, and SCD25 peaking 24–72 hours during Cycle 1 for both arms.
- In responding patients of both arms, the density of tumor infiltrating CD8+ cells increased alongside a rapid decrease in tumor burden before Cycle 2 Day 1.
- By Day 8 after first IV and SC administration:
-
- A decrease in the serum BCMA plasma levels was observed.
- All responding patients had a decrease in plasma serum BCMA by Cycle 3 Day 1.
- A rapid (<1% multiple myeloma plasma cells in 90% [31 out of 34 patients]) and deep (30% achieving MRD negativity of 10−5) clearance of MM cells upon forimtamig treatment was observed independent of baseline GPRS5D expression level.2,3
Forimtamig versus 1:1 GPRC5D-TCB and 2:1 BCMA-TCB: Preclinical results1,4
In an MM xenograft mouse model, forimtamig demonstrated higher efficacy across all tested doses when compared with talquetamab and alnuctamab despite higher BCMA expression at baseline.
In the ex vivo analysis of autologous bone marrow aspirates from newly diagnosed MM, forimtamig monotherapy showed high potency by inducing increased tumor-infiltrating lymphocyte activation (increased CD25 expression on CD4+ and CD8+ T cells). It also demonstrated high efficacy indicated by the rapid killing of tumor cells at very low doses. Combined with standard-of-care therapies, forimtamig induced higher T-cell activation and resulted in tumor cell killing in combination.
To further establish the biomarker analysis, a longitudinal analysis using an orthotopic in vivo model of MM in mice was performed.
- As early as 72 hours of injection, forimtamig was shown to induce rapid killing of tumor cells as indicated by significant reduction in soluble BCMA; this activity was independent of the baseline tumor load.
- In peripheral blood, forimtamig induced strong T-cell margination lasting 48 hours after first injection and strong T-cell expansion as early as 24 hours after second injection.
- In tumors, a 5-fold increase in CD8+ expression was observed 72 hours after first and second dosing and strong T-cell differentiation in the peripheral blood and tumor cells.
- Forimtamig induced cytokine release with peaks between 4 hours and 72 hours; peaks were cytokine specific.
- In this analysis, step-up dosing was shown to mitigate the cytokine release whilst also maintaining strong anti-tumor activity.
GPRC5D-directed CAR T-cell therapies
The Multiple Myeloma Hub previously reported key findings from two GPRC5D-directed CAR T-cell therapies; OriCAR-017 (a second-generation, autologous, GPRC5D-directed CAR T-cell therapy investigated in the phase I POLARIS study [NCT05016778] recently published in The Lancet Haematology)5 and MCARH109 (NCT04555551), which have demonstrated safety and efficacy in heavily pretreated patients with R/R MM. The first results on another GPRC5D-targeted CAR T-cell, CC-95266 (NCT04674813), were presented by Berdeja6 at ASH 2022. We report the key findings below.
Phase I trial of CC-95266
Study design
- This was a phase I open-label, multicenter, dose escalation study encompassing patients with R/R MM who received ≥3 prior regimens (including autologous stem cell transplant, a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38) and who progressed within 12 months of last treatment (except if it was CAR T-cell therapy).
- Patients were treated with CC-95266 across five dose levels (25, 75, 150, 300, and 450 × 106).
- Primary endpoints were safety, tolerability, and determination of maximum tolerated dose/recommended phase II dose.
Results
- Among the 33 patients treated, the following was seen at baseline:
- Median age was 63 years
- Median number of prior lines of therapies was 4; 66.7% received prior stem cell transplant
- 48.5% had high-risk cytogenetics
- 45.5% had extramedullary plasmacytoma
- 54.5% had prior BCMA-directed therapy
- 40% had prior BCMA-directed CAR T-cell therapy
Efficacy
- At data cut off (September 7, 2022), the overall response rate and CR was 89.5% and 47.4%, respectively, among 19 evaluable patients, with responses observed across all dose levels.
- The majority of patients (7 out of 9) receiving prior BCMA-directed therapy were treated with investigational or approved CAR T-cell treatment.
- Response rates for this patient subset versus those who did not receive prior BCMA treatment are reported in Figure 2.
- Four patients within the treated cohort who achieved a CR had a next-generation sequencing-MRD-negativity of 10−5 by Month 3.
- At the median 3.1 months of follow up, ongoing responses were observed in 15 out of 17 patients.
Figure 2. Response rates*
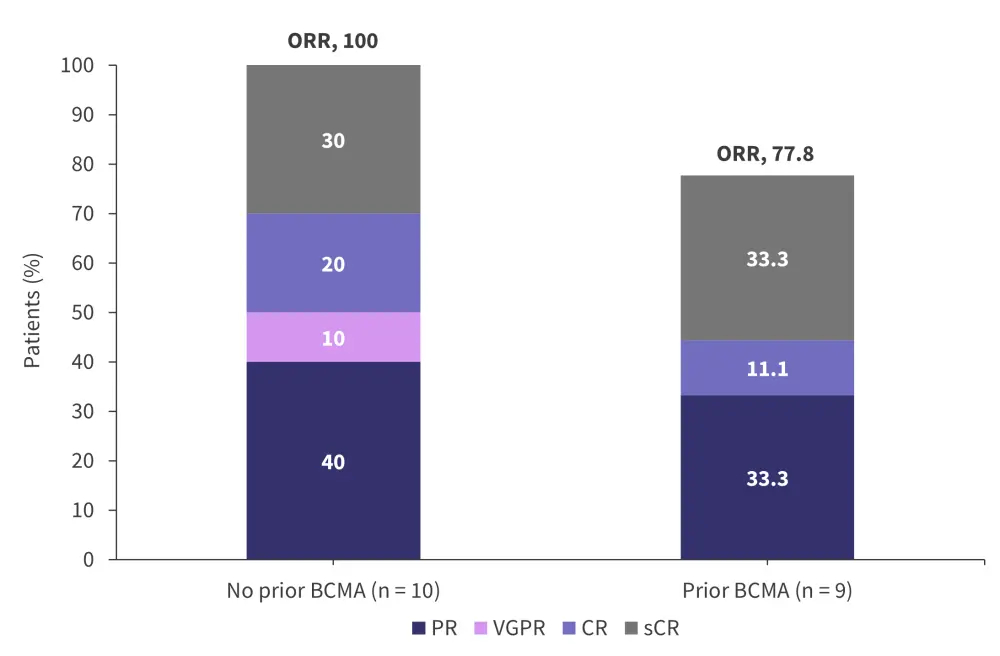
BCMA, B-cell maturation antigen; CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent CR; VGPR, very good partial response.
*Data from Berdeja.6
Safety profile
- Any grade treatment emergent adverse events occurred in 88% of treated patients; 73% were Grade 3–4.
- The most common hematologic toxicities reported were neutropenia (any grade, 66.7%; Grade 3–4, 60%) and thrombocytopenia (any grade, 40%; Grade 3–4, 21%).
- Dose limiting toxicities (prolonged Grade 4 neutropenia/thrombocytopenia) were seen in two patients at the 25 × 106 and 75 × 106 dose levels and the maximum tolerated dose has not been exceeded.
- No treatment-related deaths were reported.
The most common non-hematologic toxicity was CRS (any grade, 64%; Grade 3–4, 6%), followed by pyrexia, hypokalemia, and headache (all reported in 30% of patients). The incidence of CRS was reported across all dose levels, with no dose effect observed. The median time to CRS onset and median duration of CRS was 3 days and 4 days, respectively. The occurrence of low-grade ICANS was seen in two patients and was reversed with steroid treatment. The on-target AEs reported were all Grade 1 (skin, 30%; taste disorder, 15%; nail dysmorphia, 9%, and dysphagia, 3%) with most cases not requiring intervention.
Pharmacodynamic and pharmakinetics profile of CC-95266
Pharmacodynamic analysis of CC-95266 showed reduced soluble BCMA levels indicating reduced tumor burden across all dose levels. Pharmacokinetic profiling revealed consistent fast expansion and multiphasic decline after infusion, dose-dependent cellular expansion on increased dose up to 150 × 106, and pharmacokinetic variability at higher dose levels.
Conclusion
GPRC5D-directed bispecific antibodies have demonstrated promising results in R/R MM. The updated analysis of forimtamig demonstrated high clinical activity with high response rates, early durability, and ongoing responses observed across both SC and IV arms. The safety profile was consistent with the known forimtamig mode of action and GDRC5D target expression, with step-up dosing mitigating the risk of severe CRS.
In the biomarker analysis, forimtamig displayed T-cell engagement and rapidly effective T-cell mediated antitumor activity across both routes of administration, with SC administration resulting in a delayed cytokine release that could offer a safety benefit in patients with R/R MM.
These data confirm the preclinical findings, which showed the high potency and efficacy of forimtamig as both a monotherapy and in combination with standard-of-care therapies, and the importance of step-up dosing for managing CRS. Investigations to optimize SC and IV dosing of forimtamig are ongoing.
Similarly, GPRC5D-targeted CAR T-cell therapies have revealed encouraging results. Preliminary data on the directed CAR T-therapy CC-95266 showed a favorable safety profile, with no high-grade CRS or ICANS neurotoxicity reported. Efficacious responses were also observed in patients treated with prior BCMA-directed treatments, with MRD-negative CRs achieved in some patients.
Based on these data, GPRC5D-directed CAR T-cells and bispecific antibodies remain promising regimens for the treatment of patients with R/R MM.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

