All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Efficacy of OriCAR-017, a GPRC5D-directed CAR T-cell therapy, in patients with R/R MM: Findings from the POLARIS study
Chimeric antigen receptor (CAR) T cells have shown some promising results in multiple myeloma (MM). However, first-generation CAR T-cell therapies that target B-cell maturation antigen (BCMA) have shown varied responses, including relapse in some patients.1,2
Another potential target, G protein-coupled receptor family C group 5 member D (GPRC5D), is an orphan surface receptor with high and selective expression on myeloma cells; however, its expression on normal tissues is restricted to hair follicles in humans. GPRC5D expression is also independent of BCMA expression, making it an ideal target for patients with relapsed/refractory (R/R) MM with or without prior BCMA-target therapy. Its expression is limited in normal tissues, which reduces the adverse effects of GPRC5D-directed CAR T-cell therapy.1,2 Early analyses, which suggest GPRC5D is a suitable target for CD3-mediated T-cell redirection, have previously been summarized by the Multiple Myeloma Hub.
OriCAR-017 is a second-generation, autologous, GPRC5D-directed CAR T-cell therapy that improves the expansion and durability of CAR T-cells post-transfusion.1,2 The results of the phase I POLARIS study (NCT05016778) of OriCAR-017 in heavily pretreated patients with R/R MM were recently presented during the European Hematology Association (EHA) 2022 Congress by He Huang1 and during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting by Jiasheng Wang. 2 We summarize the key findings here.
Study design1,2
POLARIS is a phase I, open-label, single-center, dose escalation trial in heavily pretreated patients with R/R MM who had a measurable disease as per the International Myeloma Working Group (IMWG) criteria, GPRC5D expression >20% on plasma cells, were previously treated with ≥3 therapeutic options with different mechanisms, and were enrolled between June 2021 and April 2022. Prior BCMA-targeted therapy was allowed. Eligible patients underwent apheresis and lymphodepletion followed by a dose escalation phase with a single infusion of OriCAR-017, as shown in Figure 1.
Figure 1. Treatment schema*
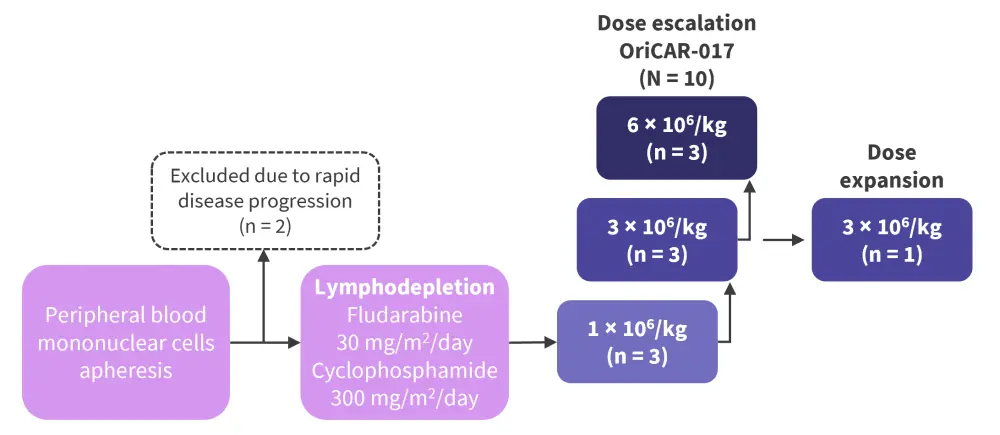
*Adapted from Huang1 and Wang.2
The primary endpoints were safety and tolerability measured by dose-limiting toxicity developed from the time of initial treatment to Day 28 post-infusion, adverse events (AEs) assessed using the Common Terminology Criteria for AEs (CTCAE), and serious AEs. Secondary endpoints were efficacy and pharmacokinetics measured by the concentration of CAR T cells, overall response rate, and progression-free survival.
Baseline characteristics
A total of 10 patients were included, with a median age of 64 years (range, 14–71 years) and an equal distribution of male (50%) and female (50%) patients. Of note, 50% of patients were previously treated with BCMA therapy (Table 1).
Table 1. Baseline characteristics*
|
Auto-HSCT, autologous hematopoietic stem cell transplantation; BCMA, B-cell maturation antigen; CAR T-cells, chimeric antigen receptor T-cells; ECOG PS, Eastern Cooperative Oncology Group performance status; GPRC5D, G protein-coupled receptor family C group 5 member D; ISS, International Staging System. |
|
|
Characteristic, % (unless otherwise stated) |
All patients (N = 10) |
|---|---|
|
ECOG PS |
|
|
0 |
10 |
|
1 |
30 |
|
2 |
60 |
|
≥1 extramedullary plasmacytosis |
40 |
|
ISS stage |
|
|
I |
30 |
|
II |
40 |
|
III |
30 |
|
Cytogenetic risk |
|
|
High |
60 |
|
Standard |
10 |
|
Unknown |
30 |
|
≥50% GPRC5D expression |
80 |
|
Prior therapies, median (range) |
5.5 (3–17) |
|
Proteasome inhibitors |
|
|
Ixazomib |
50 |
|
Carfilzomib |
10 |
|
Bortezomib |
100 |
|
Immunomodulatory drugs |
|
|
Lenalidomide |
100 |
|
Thalidomide |
50 |
|
Pomalidomide |
40 |
|
Anti-CD38 monoclonal antibodies |
20 |
|
Auto-HSCT |
20 |
|
BCMA CAR T-cells |
50 |
Results
The data cutoff point was April 30, 2022.
Safety
Dose-limiting toxicities, immune effector cell-associated neurotoxicity syndrome, serious AEs, and deaths due to AEs were not observed.1,2 Three or more hematologic toxicities occurred following lymphodepletion.1 The most common treatment-emergent AEs were Grade 3 or 4 hematologic toxicities, including neutropenia (100%), leukopenia (90%), thrombocytopenia (90%), and anemia (70%).2 Cytokine release syndrome (CRS) was observed in all patients, with 90% and 10% of patients experiencing Grade 1 and 2 CRS, respectively.1,2 The median time to CRS onset was 2 days (range, 1–9 days) and the median duration of CRS was 6 days (range, 3–9 days).1,2
Pharmacokinetics
OriCAR-017 showed robust expansion in peripheral blood as well as bone marrow at all three dose levels. The median peak expansion for all three dose levels in peripheral blood was 10 days (range, 7–14 days) (Table 2).2
Patients at dose level 3 × 106/kg performed significantly better in terms of persistence of CAR T cells compared with the other dose levels at Month 3; therefore, this dose level was selected by investigators for the dose expansion cohort.2
Table 2. Pharmacokinetics in peripheral blood*
|
AUC, areas under curve; Cmax, maximum concentration Tmax, time to maximum concentration. |
|||
|
Pharmacokinetic parameter |
Dose |
||
|---|---|---|---|
|
1 × 106/kg |
3 × 106/kg |
6 × 106/kg |
|
|
Median Tmax, day (range) |
10 (7–14) |
10 (7–14) |
10 (7–14) |
|
Median Cmax, copies/µl (range) |
19,424 |
10,149 |
5,610 |
|
AUC0–28d, copies/µl × day (range) |
187,000 |
91,550 |
75,800 |
Efficacy1,2
At a median follow-up of 175 days (range, 35–281 days), responses assessed by investigators were observed in all patients at all dose levels with a 100% overall response rate and 60% complete response and stringent complete response rate (Table 3). These responses were deep and durable, including in BCMA-relapsed patients (n = 5). At data cutoff, all patients showed progression-free survival and were followed without additional therapy.
Table 3. Investigator-assessed response rates*
|
CR, complete response; MR, minimal response; ORR, overall response rate; PD, progressive disease; PR, partial response sCR, stringent CR; SD, stable disease; VGPR, very good partial response. |
||||
|
Response, % |
Dose |
Total |
||
|---|---|---|---|---|
|
1 × 106/kg |
3 × 106/kg |
6 × 106/kg |
||
|
sCR |
100 |
75 |
0 |
60 |
|
CR |
0 |
0 |
0 |
0 |
|
VGPR |
0 |
0 |
100 |
30 |
|
PR |
0 |
25 |
0 |
10 |
|
MR |
0 |
0 |
0 |
0 |
|
SD |
0 |
0 |
0 |
0 |
|
PD |
0 |
0 |
0 |
0 |
|
ORR |
100 |
100 |
100 |
100 |
At Day 28, all patients achieved minimal residual disease (MRD)-negative status in bone marrow measured by flow cytometry (sensitivity, 10−5). Nine patients continued to be MRD-negative at Month 3, five patients at Month 6, and one patient at Month 9 after infusion (Figure 2).
Figure 2. Patients with MRD-negative status at different timepoints following infusion*
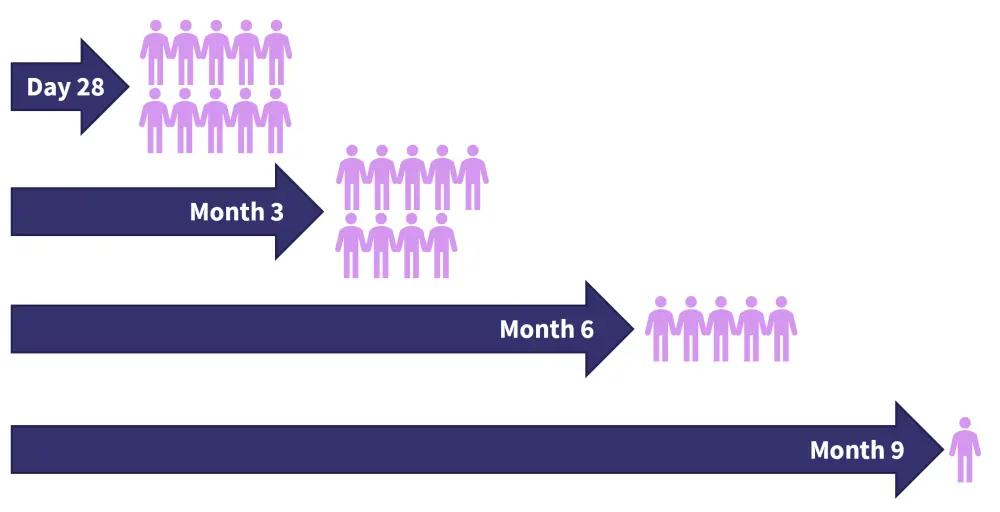
MRD, minimal residual disease.
*Adapted from Huang1 and Wang.2
Conclusion
This phase I study shows a notable safety profile and efficacy of OriCAR-017 in heavily treated patients with R/R MM. Most treatment-emergent AEs observed were low grade, manageable, and reversible. Responses were rapid, deep, and durable at all dose levels, including in those with prior BCMA-targeted CAR T-cell therapy. All patients achieved MRD-negative status with a tolerable safety profile, indicating the potential of OriCAR-017 as an alternative therapy choice in patients with R/R MM. Patients with BCMA CAR T-cell therapy relapse also benefitted from OriCAR-017.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

