All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Talquetamab for relapsed/refractory multiple myeloma: Results from a phase I study
Featured:
B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T-cell therapy has demonstrated encouraging clinical responses in patients with multiple myeloma (MM). However, differential BCMA expression leads to varied responses and, in some cases, relapse. It is therefore of interest to identify alternative targets for T-cell redirection for the treatment of relapsed/refractory MM (RRMM).
Introduction
G protein-coupled receptor family C group 5 member D (GPRC5D) has been highlighted as an attractive target in MM. GPRC5D is an orphan receptor of unknown function, and its expression in humans is restricted to hair follicles. However, early analyses have suggested that GPRC5D mRNA and protein expression levels are elevated in plasma cells in patients with MM, monoclonal gammopathy of undetermined significance, and smoldering MM.1,2 Furthermore, heightened GPRC5D has been associated with translocation t(4;14), poor overall survival, and unfavorable prognosis.2 The absence of GPRC5D on healthy human tissue, alongside the increased expression in disease, makes it a viable target for exploration in the MM setting.
The structure of GPRC5D is also beneficial when considering T-cell redirecting therapy, providing further justification for its exploration. As a seven-pass membrane protein, the likelihood of GPRC5D shedding into serum is low. Protein shedding is associated with a phenomenon known as the ‘sink effect’, which has been observed with BCMA-targeted therapies and results in reduced efficacy.1 Moreover, because GPRC5D expression is independent of BCMA, it could also be a promising target for patients who relapsed from BCMA-directed therapy due to antigen loss.3
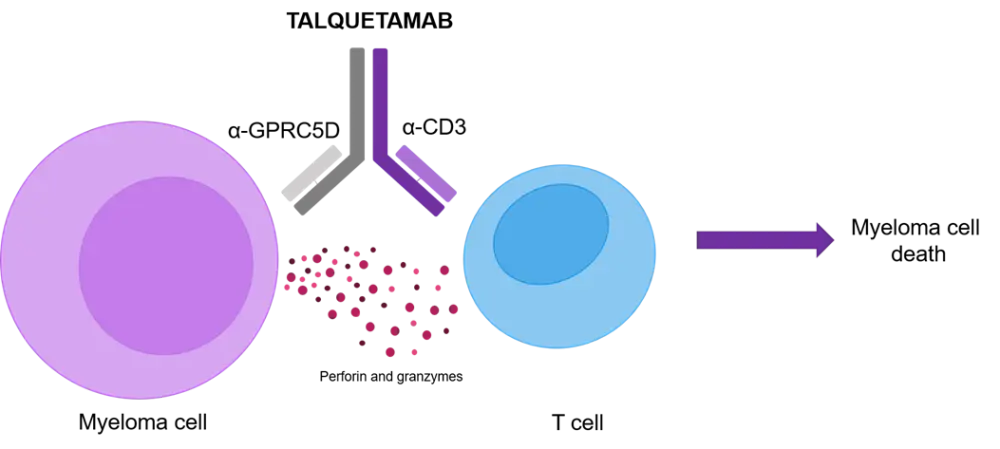
All things considered, GPRC5D has been proposed as a suitable target for CD3-mediated T-cell redirection, resulting in the design of an anti-GPRC5D × CD3 bispecific antibody. The first-in-class bispecific antibody, talquetamab (Figure 1), facilitates the interaction of T-cells and GPRC5D-expressing myeloma cells and subsequent MM cell death. Preclinical and xenograft studies have uncovered the promising anti-tumor activity of talquetamab. An additional asset of talquetamab is its pharmacokinetic (PK) profile, which may reduce the frequency of subcutaneous dosing and, therefore, positively impact the patient experience.1
Figure 1. Design of anti-GPRC5D × CD3 bispecific antibody talquetamab (adapted from Chari et al.1)
To explore the feasibility of talquetamab in the RRMM setting, a first-in-human phase I study of talquetamab in patients with RRMM was conceived (NCT03399799). The results were presented by Ajai Chari during the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition1 and are summarized below.
Study design1
Patients with measurable MM who were relapsed/refractory or intolerant to established MM therapies were eligible for enrollment. Patients may have been exposed to prior BCMA-targeted therapies and had to meet the following blood count criteria:
- Hemoglobin ≥ 8 g/dL
- Platelets ≥ 50 × 109/L
- Absolute neutrophil count ≥ 1.0 × 109/L
The primary objectives of the study were to
- establish the recommended phase II dose (RP2D);
- evaluate the safety and tolerability of talquetamab at the RP2D; and
- determine the antitumor activity and the PK and pharmacodynamic profiles of talquetamab in patients with RRMM.
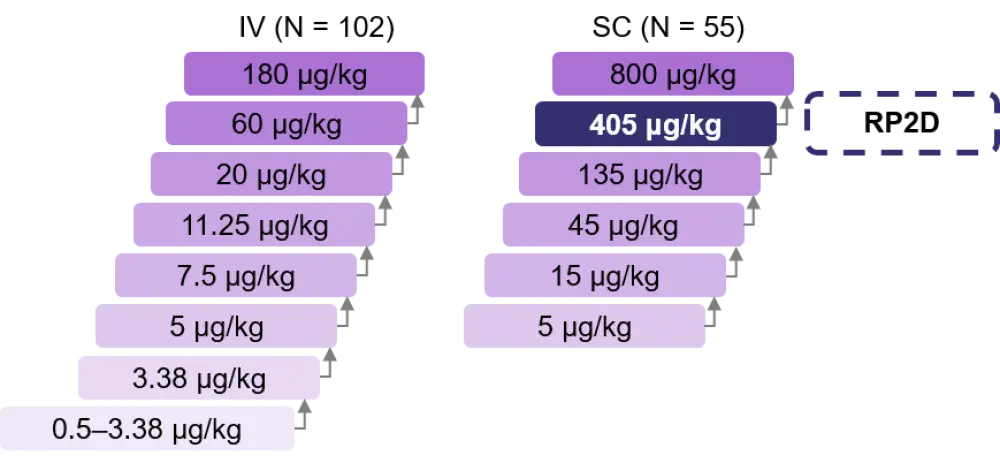
Patients received talquetamab weekly (QW) or every 2 weeks (Q2W) either with or without step up dosing as shown in Figure 2. Pretreatment with a glucocorticoid, antihistamine, and antipyretic was required for step up dosing and prior to the first full dose. Following the first full dose, steroid treatment was not required.
Figure 2. Phase I step up dosing design (adapted from Chari et al.1)
Results1
- RP2D: 405 μg/kg talquetamab, subcutaneous (SC).
- Patient characteristics are outlined in Table 1.
Table 1. Baseline patient characteristics1
|
BCMA, B-cell maturation antigen; RP2D, recommended phase II dose; SC, subcutaneous. |
||
|
Total |
405 μg/kg talquetamab SC |
|
|---|---|---|
|
Median age, years (range) |
64 (33–80) |
61 (49–80) |
|
Male |
57 |
58 |
|
Bone marrow plasma cells ≥ 60% |
22 |
19 |
|
Extramedullary plasmacytomas ≥ 1 |
20 |
37 |
|
High-risk cytogenetics* |
13 |
6 |
|
Median prior lines of therapy, n (range) |
6 (2‒20) |
4.5 (2‒14) |
|
Prior transplantation |
86 |
79 |
|
Prior anti-BCMA therapy |
17 |
16 |
|
Refractory status |
|
|
|
Carfilzomib |
67 |
58 |
|
Pomalidomide |
76 |
79 |
|
Anti-CD38 |
95 |
95 |
|
Triple-class |
82 |
68 |
|
Penta-drug |
33 |
21 |
|
Refractory to last line of therapy |
87 |
79 |
Safety
- No dose-limiting toxicities were observed at the RP2D, but three were seen across the entire study.
- Dose reductions were less common in patients who received the RP2D and occurred later when compared with patients who received 800 μg/kg SC talquetamab.
- The most common adverse events (AEs) are shown in Table 2, other events of interest are noted below.
- Across all doses, 38% of patients experienced an infection (8% were Grade ≥ 3). In patients who received the RP2D, this reduced to 16% and no Grade ≥ 3 infections were observed.
- Across all doses, 6% of patients experienced neurotoxicity. In patients who received the RP2D, this reduced to 5% and no Grade ≥ 3 neurotoxicity was observed with SC administration at any dose.
- Across all doses vs patients who received the RP2D
- 18% vs 21% experienced injection site reactions. All were Grade 1–2.
- 45% vs 58% experienced skin-related AEs. Most events were Grade 1–2.
- 17% vs 21% experienced nail disorders.
- No Grade 5 AEs were observed at any dose.
Table 2. AEs observed in ≥25% of patients who received talquetamab1
|
AE, adverse event; CRS, cytokine release syndrome; RP2D, recommended phase II dose; SC, subcutaneous. |
||||
|
Grade ≥ 3 AEs, % |
Total |
405 μg/kg talquetamab SC |
||
|---|---|---|---|---|
|
|
All Grade |
Grade ≥ 3 |
All Grade |
Grade ≥ 3 |
|
Hematologic |
|
|
|
|
|
Anemia |
48 |
27 |
26 |
0 |
|
Neutropenia |
47 |
31 |
47 |
42 |
|
Lymphopenia |
40 |
36 |
16 |
16 |
|
Leukopenia |
32 |
16 |
21 |
16 |
|
Thrombocytopenia |
32 |
13 |
21 |
5 |
|
Nonhematologic |
|
|
|
|
|
CRS |
54 |
3 |
68 |
0 |
|
Dysgeusia |
38 |
NA |
47 |
NA |
|
Fatigue |
29 |
1 |
16 |
0 |
|
Headache |
27 |
1 |
16 |
0 |
|
Pyrexia |
27 |
1 |
11 |
0 |
|
Diarrhea |
25 |
3 |
16 |
0 |
Cytokine release syndrome
- Overall, cytokine release syndrome (CRS) of any grade was observed in 54% of patients.
- Of the patients who received RP2D talquetamab, 68% experienced Grade 1 (52%) or Grade 2 (15%) CRS. No patients in this group experienced Grade 3 CRS.
- The only Grade 3 CRS cases were observed in patients who received talquetamab IV.
Efficacy
- Median follow-up was 3.7 months.
- The median time to first response was 1 month.
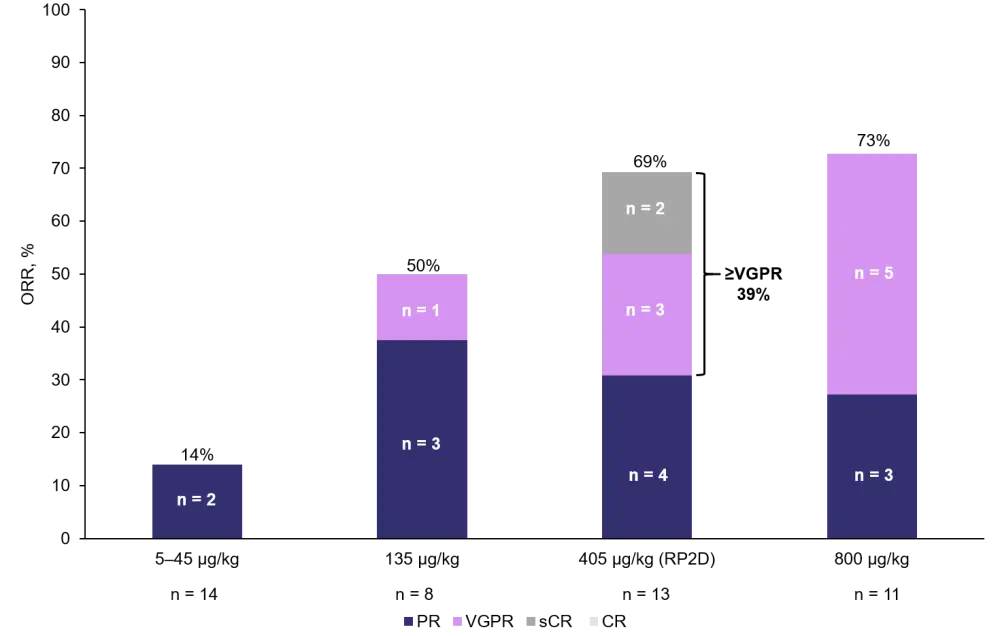
- The overall response rate (ORR) for all patients who received SC talquetamab was 66%. Responses by dose level are presented in Figure 3.
- Of the triple-class and penta-drug refractory patients, 67% (six out of nine patients) and 100% (two out of two) responded, respectively.
Figure 3. ORR in patients who received SC talquetamab (adapted from Chari et al.1)
Conclusion
GPRC5D represents a promising novel target for the treatment of MM. This study identified a RP2D of 405 μg/kg SC talquetamab, at which the first-in-class agent demonstrated encouraging safety and efficacy profiles. No Grade ≥ 3 CRS or neurotoxicity were observed in patients who received SC talquetamab at any dose. Furthermore, SC administration is more patient-friendly compared with IV, and the PK profile of talquetamab may allow for less frequent dosing. ORRs were encouraging across all patients, including those who were triple-class and penta-drug refractory, and responses improved over time. The dose expansion study is ongoing, and a phase II trial (NCT04634552) is planned to start recruiting soon.
Expert Opinion
https://multiplemyelomahub.com/medical-information/why-gprc5d-is-a-promising-target-for-multiple-myeloma
 Ajai Chari
Ajai ChariReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

