All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Forimtamig, a novel bispecific antibody targeting GPRC5D, for the treatment of RRMM
GRPC5D, a G protein-coupled receptor, is a key therapeutic target in multiple myeloma (MM) as it is highly expressed on malignant plasma cells.1 The Multiple Myeloma Hub has previously reported on talquetamab, an anti-GPRC5D bispecific antibody that has recently been granted breakthrough therapy designation by the U.S. Food and Drug Administration (FDA) for use in patients with relapsed/refractory (R/R) MM (RRMM). More information on talquetamab and GPRC5D can also be found here.
At the European Hematology Association (EHA) 2022 Congress, Hasselbalch Riley presented preliminary results from the first-in-human clinical trial of forimtamig (also known as RG6234) as a novel anti-GPRC5D T-cell engaging bispecific antibody in RRMM (NCT04557150).1 In addition, Dekhtiarenko presented supporting data that confirm the mode of action of this agent.2
Forimtamig mode of action2
Forimtamig utilizes a dual-binding mechanism, binding with high-avidity to GPRC5D on plasma cells and high-affinity to CD3 on T cells, which induces T-cell activation and killing of malignant plasma cells. Forimtamig also features a silent Fc region that reduces toxicity and increases its half-life. The configuration of forimtamig is shown in Figure 1.
Figure 1. Forimtamig configuration*
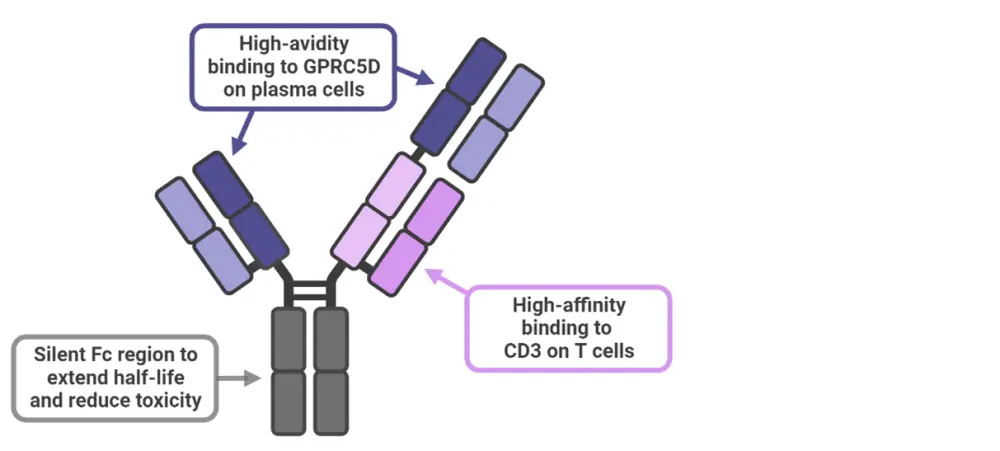
*Adapted from Dekhtiarenko.2 Created with BioRender.com
Study design1,2
In the ongoing dose escalation study, patients with RRMM were given intravenous or subcutaneous infusions of forimtamig in a step-up dosing regimen, up to a target optimal dose, followed by forimtamig every 2 weeks for 1 year. The target optimal dose varied between 18 µg and 10,000 µg among patients.
Key inclusion criteria for the study included receiving a prior immunomodulatory imide drug and proteasome inhibitor, and an Eastern Cooperative Oncology Group performance status of 0–1. Primary endpoints included safety and tolerability, and determination of the maximum tolerated dose to inform the phase II dose.
Results
Baseline characteristics1
Patient characteristics are shown in Table 1. There was a high proportion of patients with high-risk cytogenetics or triple- or penta-class refractory disease.
Table 1. Baseline patient characteristics*
|
BCMA, B-cell maturation antigen. |
|
|
Patient characteristics, % (unless otherwise stated) |
N = 51 |
|---|---|
|
Age range, years |
27–78 |
|
Male |
54.9 |
|
High-risk cytogenetics (n = 30) |
46.7 |
|
Extramedullary disease |
13.7 |
|
Median number of prior lines of therapy† |
5 |
|
Triple-class refractory†‡ |
62.5 |
|
Penta-class refractory†§ |
31.3 |
|
Prior anti-CD38 antibody† |
81.3 |
|
Prior anti-BCMA†¶ |
20.8 |
Efficacy1
Of the 51 patients included in the study, 42 were evaluated for efficacy, with an overall response rate of 71.4% (Figure 2). The median time to first response was 1.2 months (n = 30) and, as of April 5, 2022, responses were ongoing in 25 patients.
Figure 2. Response rate across all target doses (18–10,000 µg)
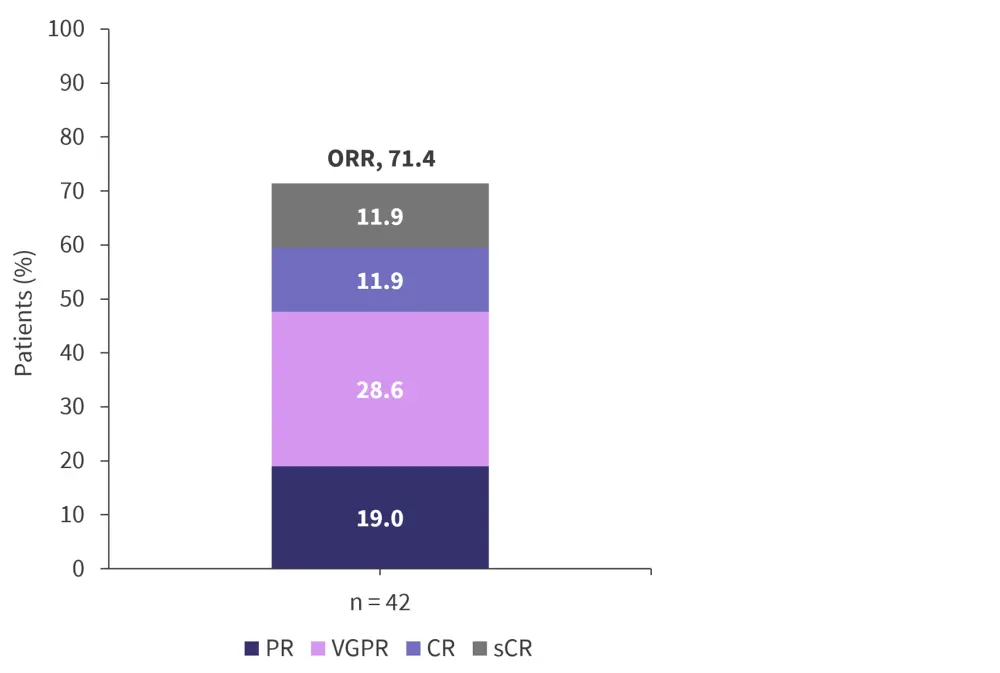
CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Hasselbalch Riley, et al.1
Safety1
The step-up dosing regimen was used to mitigate the risk of severe cytokine release syndrome (CRS). Of the 51 patients in the study, CRS of any grade occurred in 40; there was only one case of Grade 3 and no cases led to treatment discontinuation. Discontinuation due to any adverse event occurred in one patient, and there were no fatal events. Other adverse events of note are shown in Table 2.
Table 2. Any grade and Grade ≥3 adverse events*
|
GI, gastrointestinal; ICANS, immune effector cell-associated neurotoxicity syndrome. |
||
|
Adverse event, % |
Any Grade |
Grade ≥3 |
|---|---|---|
|
ICANS |
5.9 |
2.0 |
|
Skin |
64.7 |
9.8 |
|
GI epithelium or tongue |
64.7 |
0 |
|
Hair and nail changes |
15.7 |
0 |
|
Hematologic |
37.3 |
25.5 |
|
Anemia |
27.5 |
13.7 |
|
Thrombocytopenia |
25.4 |
13.7 |
|
Neutropenia |
23.6 |
11.8 |
|
Infections |
52.9 |
17.6 |
|
COVID-19 |
13.7 |
2.0 |
Biomarker analysis2
In order to determine whether forimtamig can induce T-cell killing of myeloma cells, biomarker analysis was performed on bone marrow samples using flow cytometry, serum B-cell maturation antigen and an immunoassay, and CD138/CD8 immunohistochemistry staining. These analyses demonstrated the following:
- Cytokine release occurred after all given target doses (18–10,000 µg) of forimtamig, peaking 4–24 hours after the first dose; however, the magnitude of cytokine release decreased after subsequent step-up doses.
- Following forimtamig infusion, activation of CD8+ T cells in peripheral blood occurred, peaking 4 days into the first treatment cycle.
- In the majority of responding patients (8 of 12), forimtamig induced tumor infiltrating CD8+ T cells.
- By Day 1 of Cycle 3, all patients responding to treatment showed a decrease in plasma serum B-cell maturation antigen levels.
- The efficacy of forimtamig appeared to be independent of GPRC5D expression.
- By Day 1 of Cycle 2, most patients (17 of 18) had <1% of MM cells in their bone marrow aspirate.
Conclusion
Bispecific antibodies are a promising therapeutic option for patients with R/R MM. The presenters concluded that the high response rate observed in the study, alongside the low incidence of severe CRS, demonstrates the potential of forimtamig, although they noted the small sample size limitation. As the study continues, clarification on the optimum dose and additional safety data will be obtained. Biomarker analysis revealed that forimtamig induced rapid and effective T-cell mediated anti-myeloma activity, with further trials needed to further understand the mechanism of action.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


