All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Talquetamab receives breakthrough therapy designation from the FDA
On the June 29, 2022, talquetamab was granted breakthrough therapy designation by the U.S. Food and Drug Administration (FDA) for use in patients with relapsed/refractory multiple myeloma.1 At the European Hematology Association (EHA)2022 Congress, updated safety and efficacy data from the phase I/II MonumenTAL-1 trial (NCT03399799 and NCT04634552) were presented by Monique Minnema,2 and we are pleased to provide a summary of this presentation here.
Talquetamab is an off-the-shelf bispecific antibody that targets GPRC5D on myeloma cells and CD3 on T cells. One of the benefits of this drug is that it is administered subcutaneously rather than intravenously, which can be less distressing for patients. The Multiple Myeloma Hub has previously reported on the characteristics of GPRC5D and the mechanism of action of talquetamab; you can find the article here.
Latest data from the MonumenTAL-1 trial
The updated results of the MonumenTAL-1 trial include a longer follow-up of patients treated with talquetamab at the recommended phase II dose (405 µg/kg), with additional data from patients dosed at 800 µg/kg. Key patient characteristics are shown in Table 1.
Table 1. Patient characteristics*
|
ADC, antibody–drug conjugate; BCMA, B-cell maturation antigen; BsAb, bispecific antibody; IMiD, immunomodulatory drug; ISS, International Staging System; mAb, monoclonal antibody; PI, proteasome inhibitor; Pts, patients. |
||
|
Characteristic, % (unless otherwise stated) |
Pts dosed at 405 µg/kg |
Pts dosed at 800 µg/kg |
|---|---|---|
|
Median age (range), years |
61.5 (46–80) |
64 (47–84) |
|
Male |
63.3 |
47.7 |
|
High-risk cytogenetics |
11.1 |
22.5 |
|
ISS stage |
|
|
|
I |
41.4 |
37.2 |
|
II |
44.8 |
41.9 |
|
III |
13.8 |
20.9 |
|
Median prior lines of therapy (range), n |
6 (2–14) |
5 (2–17) |
|
Prior stem cell transplantation |
90.0 |
75.0 |
|
Refractory status |
|
|
|
Triple-class† |
76.7 |
77.3 |
|
Penta-drug‡ |
20.0 |
27.3 |
|
BCMA-targeted ADC or BsAb |
16.7 |
15.9 |
Safety
A step-up dosing regimen was followed to mitigate against severe cytokine release syndrome (CRS). Patients were given 2–3 step-up doses prior to the first full dose. In addition, a glucocorticoid, antihistamine, and antipyretic were given as premedication prior to the step-up doses and first full dose.
- The most common hematologic adverse events (AEs) of any grade were as follows:
- Neutropenia, which occurred in 66.7% of patients dosed at 405 µg/kg and 40.9% of patients dosed at 800 µg/kg.
- Anemia, which occurred in 56.7% of patients dosed at 405 µg/kg and 47.7% of patients dosed at 800 µg/kg.
- Other cytopenias, including lymphopenia, leukopenia, and thrombocytopenia, which occurred at similar incidence rates and were mostly confined to initial cycles.
- Any grade CRS occurred in 76.7% of patients dosed at 405 µg/kg and 79.5% of patients dosed at 800 µg/kg.
- Other frequent nonhematologic AEs of any grade were skin-related, dysgeusia, dry mouth, and nail- and rash-related.
- Any grade infections occurred in 46.7% of patients dosed at 405 µg/kg and 38.6% of patients dosed at 800 µg/kg.
- No deaths due to drug-related AEs were recorded.
Efficacy
- The median follow-up was 13.2 months for the patients dosed at 405 µg/kg and 7.7 months for the patients dosed at 800 µg/kg.
- The overall response rate (ORR) was comparable across both dosing cohorts, with an ORR of 70.0% and 63.6% for 405 µg/kg and 800 µg/kg, respectively. Response rates are shown in Figure 1.
- To date, the median duration of response has been reported to be 10.2 months and 13.0 months for the 405 µg/kg and 800 µg/kg cohorts, respectively; however, this trial is ongoing and responses that deepen over time have been recorded.
- Significant response rates have also been reported in the triple-drug and penta-class refractory subgroups at both dose levels.
- At 405 µg/kg, the ORR was 65.2% and 67.6% for triple-drug- and penta-class-refractory patients, respectively
- At 800 µg/kg, the ORRs were higher, with 83.3% of patients with triple-drug refractory disease and 75.0% of patients with penta-class refractory disease achieving at least a partial response.
Figure 1. Overall response rates for patients treated with 405 µg/kg or 800 µg/kg talquetamab*
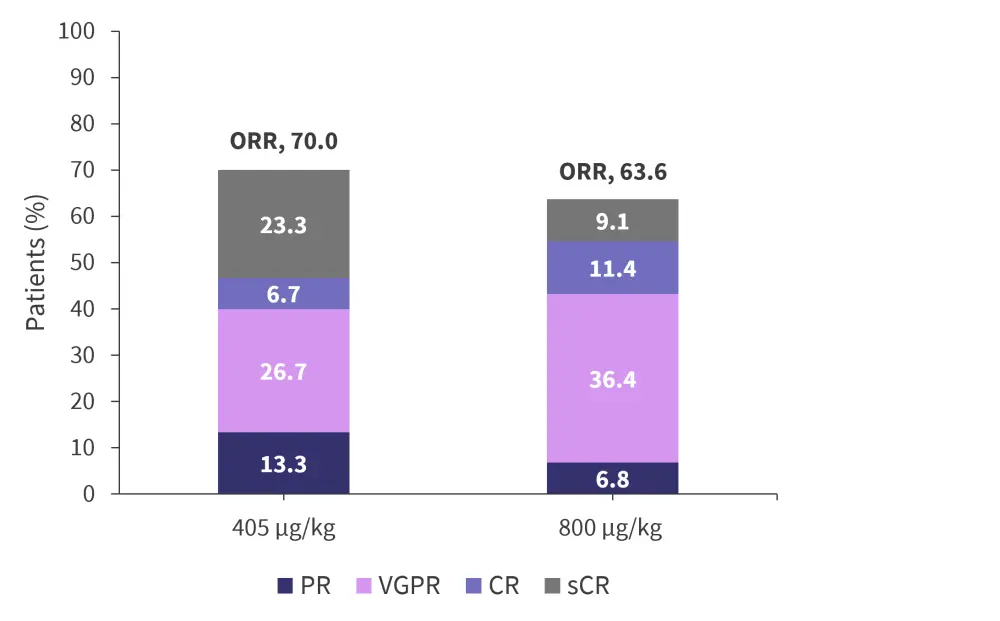
CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Minnema, et al.2
Conclusion
The updated results from this study show a promising safety and efficacy profile of talquetamab at the doses of 405 µg/kg and 800 µg/kg. Only one case of Grade 3 CRS was reported at the dose of 405 µg/kg, and no cases of Grade 3 CRS were reported with 800 µg/kg. This, alongside the promising ORRs observed, suggests talquetamab could be used for patients with relapsed/refractory multiple myeloma who have progressed to multiple lines of therapy, including patients previously exposed to B-cell maturation antigen-directed agents.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



