All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Targeting XPO1 for the treatment of MM: Real-world evidence
Do you know... Understanding the typical timing of adverse event (AE) onset during treatment for multiple myeloma is essential for effective monitoring and early management. At what point after initiating therapy are selinexor-related AEs most likely to occur?
During the Multiple Myeloma Hub Steering Committee Meeting in April 2025, key opinion leaders met to discuss exportin 1 (XPO1) as a target for the treatment of multiple myeloma (MM), with a focus on real-world evidence. The meeting opened with a presentation by Paul Richardson and featured a discussion including María-Victoria Mateos, Hang Quach, and Morie Gertz.
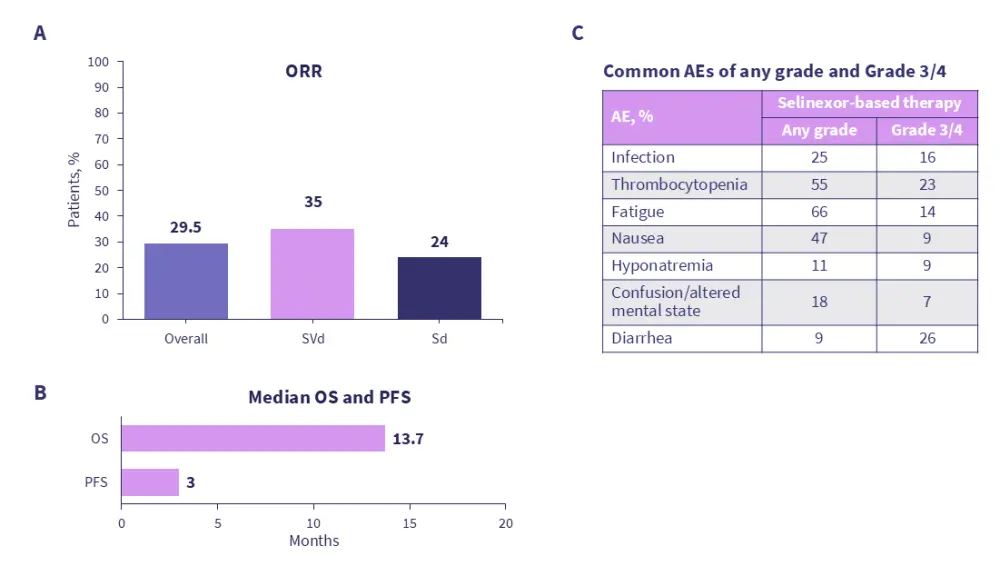
In the presentation, Richardson discussed the role of XPO1 as a target for the treatment of relapsed/refractory MM. Richardson discussed the mechanism of action for XPO1 inhibitors (Figure 1), as well as clinical trial- and real-world data (Figure 2) associated with selinexor, a first-in-class selective inhibitor of nuclear export (SINE), in heavily pretreated patient populations. During the discussion, the steering committee members provided insight into combination regimens, strategies to manage toxicities, and the potential positioning of selinexor in current treatment algorithms for MM.
Targeting XPO1 for the treatment of MM: Real-world evidence
Targeting XPO1 for the treatment of MM: Real-world evidence
Figure 1. Mechanism of action of XPO1 inhibitors
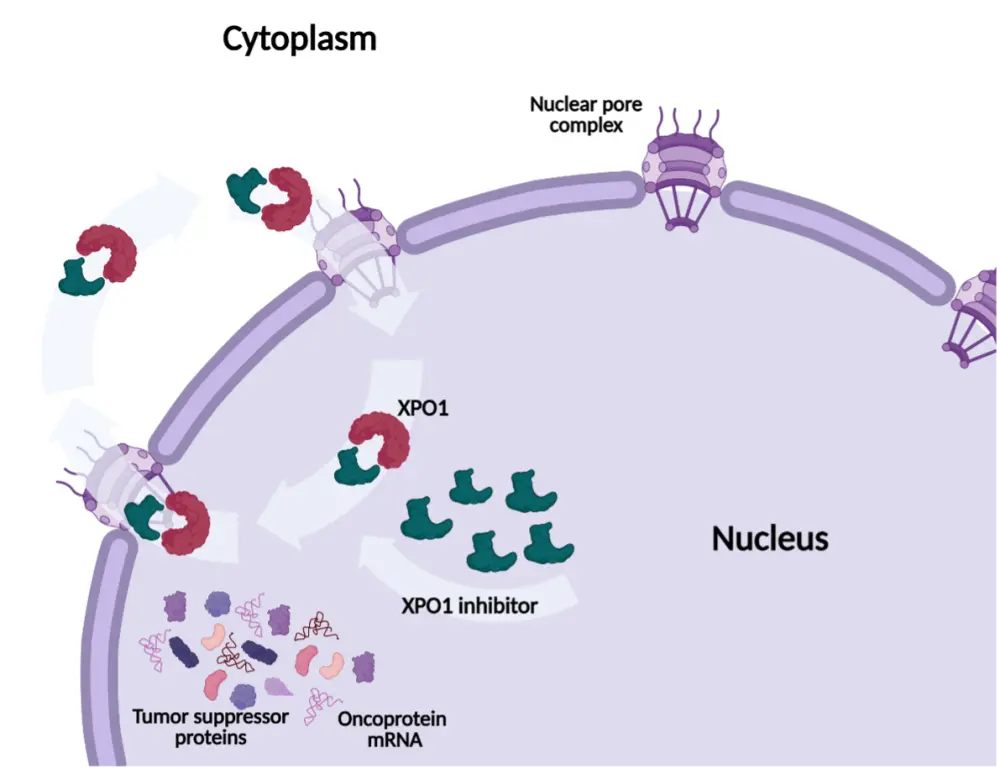
Figure 2. Real-world A response, B survival, and C adverse events associated with selinexor-based regimens*
Key learnings
Selinexor is a nuclear export inhibitor that selectively targets XPO1, a key pathway involved in myeloma cell survival.
The mechanism of action for selinexor supports its use in RRMM, particularly in patients who are refractory to proteasome inhibitors, immunomodulatory agents, and anti-CD38 monoclonal antibodies.
Selinexor-based regimens offer a clinical benefit in triple-class refractory disease, where therapeutic options are often limited.
The management of adverse effects (AEs) such as nausea, fatigue, and cytopenias is critical for ensuring patient adherence and treatment success; supportive care measures and dose adjustments are recommended.
Selinexor-related AEs are significantly more likely to occur in the first 30 days of treatment, underscoring the importance of proper monitoring and intervention, particularly at treatment initiation.3
Once-weekly dosing schedules for selinexor can help to improve the patient experience by reducing adverse effects compared with more frequent dosing.
Prophylactic antiemetics and appetite stimulants are recommended to manage gastrointestinal toxicities, particularly nausea, and improve patient experience.
Thrombocytopenia is commonly observed following treatment with selinexor and must be monitored closely. Supportive care measures include platelet monitoring and the use of agents such as thrombopoietin receptor agonists where needed.
Selinexor may offer a useful bridging therapy for patients waiting for CAR T-cell therapy or other cellular treatments.
The oral administration of selinexor offers logistical advantages for patients, particularly those needing outpatient treatment options.
Real-world experience with selinexor has helped optimize its use through improved dosing strategies and toxicity management protocols.
Treatment with selinexor-based regimens must be tailored to the individual patient’s prior treatment history and disease characteristics.
This educational resource is independently supported by Karyopharm. All content was developed by SES in collaboration with an expert steering committee; funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Paul Richardson
Paul Richardson Morie Gertz
Morie Gertz María-Victoria Mateos
María-Victoria Mateos Hang Quach
Hang Quach

