All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Targeting CD38 in MM: Challenges and resistance mechanisms
Do you know... What mechanism leads to resistance in CD38-mediated antibody-dependent cellular cytotoxicity (ADCC)?
CD38 antibody therapies1,2
CD38 is a transmembrane glycoprotein, highly expressed on malignant plasma cells, and has been established as an important therapeutic target for multiple myeloma (MM). Currently, two CD38 antibody therapies are approved for the treatment of MM1:
Daratumumab – approved as part of a combination regimen in newly diagnosed MM and as a sole agent in relapsed/refractory (RRMM)
Isatuximab – approved as part of a combination regimen in newly diagnosed MM and as part of a combination regimen in RRMM
Daratumumab and isatuximab both have proven efficacy in clinical trials and real-world treatment, confirming immunotherapy with CD38 antibodies as an effective approach for patients with MM either as a single agent or in combination with standard of care.2
Mechanisms of action2,3
CD38-directed therapies work through multiple mechanisms, including:
Complement-dependent cytotoxicity (CDC)
Antibody-dependent cell-mediated cytotoxicity (ADCC)
Antibody-dependent cellular phagocytosis (ADCP)
Apoptosis
Direct effects
Immunomodulatory effects
These mechanisms may contribute to the development of resistance to CD38 antibodies.
Impact of prior treatment
Development of resistance to treatments such as monoclonal antibodies, immunomodulatory drugs (IMiDs), and protease inhibitors (PIs) continues to pose a challenge in the treatment of MM. Management of patients with resistance to CD38 antibodies relies on novel therapies targeting different antigens, such as B-cell maturation antigen or G protein–coupled receptor, class C, group 5, member D.1,4 There are also some evidence to support retreatment with previous therapies.5 For example, a retrospective analysis of retreatment with daratumumab and pomalidomide–dexamethasone in heavily pretreated patients with RRMM, previously refractory to pomalidomide and daratumumab, gave a 33% response.5 However, use of this approach depends on the patient’s prior response and duration of treatment-free intervals.2
Primary resistance vs acquired resistance
Primary resistance occurs when myeloma cells are inherently resistant to CD38-targeting therapies. CD38 primary resistance may result from low baseline levels of CD38 expression on myeloma cells and elevated levels of complement inhibitors such as CD55 and CD59, which reduce the efficacy of CDC.2,3
Prolonged exposure to CD38-targeted therapies causes acquired CD38 resistance as it downregulates CD38 expression on myeloma cells and alters immune effector cells, including reduced activity of natural killer (NK) cells. In addition, changes in the tumor microenvironment and immune suppression can also contribute to acquired resistance.2
Mechanisms of resistance
The Multiple Myeloma Hub has previously covered resistance mechanisms for immunotherapies. The mechanism of resistance to CD38 is complex and involves multiple factors, as demonstrated in Figure 1.
Figure 1. Mechanisms of resistance to CD38 antibodies and strategies to address them*2
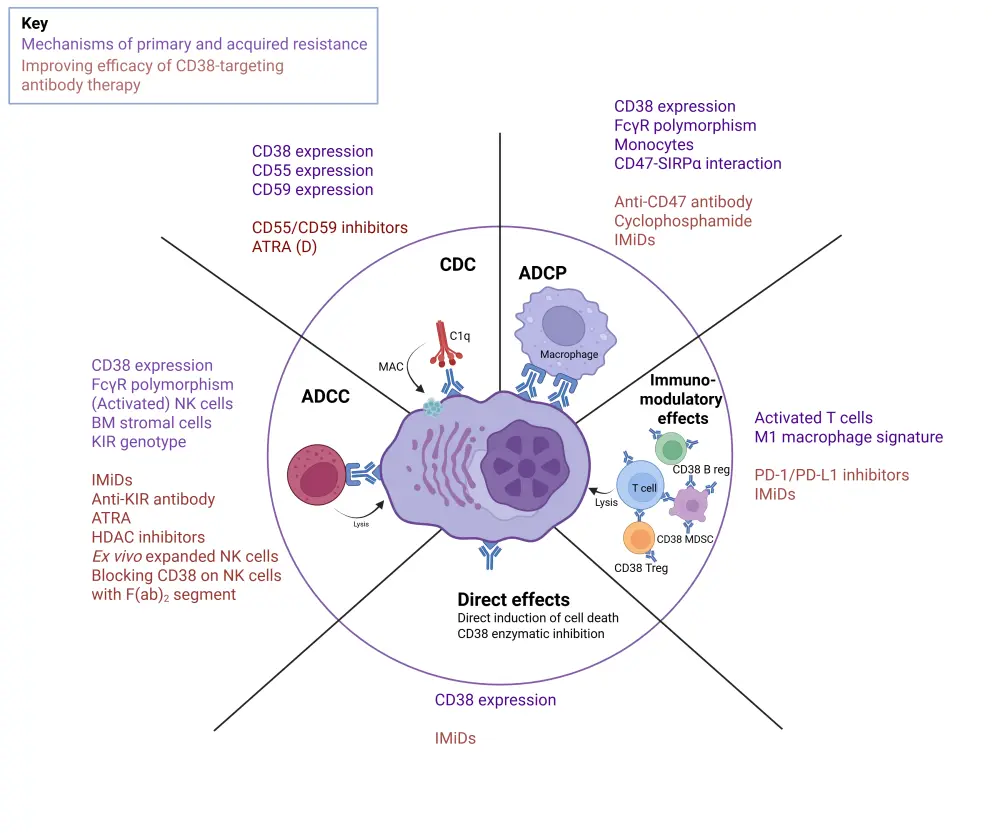
Decreased CD38 expression: Exposure to anti-CD38 monoclonal antibodies causes rapid reduction in CD38 expression, which compromises CDC and ADCC activity.1,2
CDC resistance: In patients with progressive disease, treatment with daratumumab monotherapy results in overexpression of CD55/CD59 in myeloma cells, inhibiting CDC.1,2
ADCC resistance: Through upregulation of the anti-apoptotic protein survivin, bone marrow stromal cells shield myeloma cells from being killed, including by daratumumab-mediated ADCC. The agents lenalidomide and pomalidomide, which activate NK cells, amplify daratumumab-mediated ADCC. However, daratumumab impairs malignant cell death by depleting NK cells through NK-mediated cytotoxicity.2
ADCP resistance: CD47 on myeloma cells inhibits phagocytosis via ligation of SIPRα, increasing clinical CD38 activity and impairing the tumor microenvironment. CD38 expression levels are critical for anti-CD38 monoclonal antibody-mediated ADCP.2
Fragment crystallizable gamma receptor (FcγR) polymorphism: FcγR is crucial in mediating ADCC and ADCP. FcγR polymorphism can influence the affinity of the allelic variants of FcγRs for certain immunoglobulins, thereby reducing the activation of effector cells such as macrophages and NK cells.3
Resistance to direct effect: The efficacy of anti-CD38 antibodies depends significantly on CD38 expression levels; however, the mechanism behind acquired resistance to the direct cytotoxic effects remains unclear.2
Resistance to immunomodulatory activity: Patients with RRMM who receive daratumumab monotherapy show a decrease in active T cells, which results in resistance.6 The development of resistance to the immunomodulatory effects of CD38 antibodies may also be influenced by the compensatory upregulation of different inhibitory immune checkpoint proteins associated with resistance to programmed cell death-1 or programmed death ligand-1 inhibitors.2
Cytogenetic abnormalities: Mutations and splice variants found in resistant myeloma cells impair anti-CD38 antibody binding, limiting the ability of daratumumab and isatuximab to effectively target the myeloma cells.1,2,3
Future directions
An in-depth understanding of the CD38 resistance mechanisms, including factors that contribute to the heterogeneity in responses to CD38 antibodies, will help optimize and individualize treatments for patients with MM who have developed CD38 resistance. As such, CD38 antibodies are a promising therapeutic strategy for patients with MM and CD38 antibodies currently under development for the treatment of MM include:
- MOR202 (NCT01421186)
- TAK-079 (NCT03984097)
- SAR442085 (NCT04000282)
- CID-103 (NCT04758767)
- FTL004
Combination therapies1,2,3
The combination of CD38 monoclonal antibodies with IMiDs is an approach to overcome CD38 resistance. IMiDs induce NK-cell activation, which upregulates CD38 on MM cells and enhances the cytotoxic effect of CD38 antibodies.1 Improved understanding of mechanisms that contribute to primary and acquired resistance has resulted in several new combination therapies being evaluated. For example, IMiDs have demonstrated improvements in daratumumab-mediated ADCC in lenalidomide-refractory myeloma cells, while pomalidomide enhances isatuximab-induced ADCC.1 The proteasome inhibitors carfilzomib and bortezomib have also been evaluated in combination with CD38 antibodies, although their exact mechanism of efficacy remains unclear.1 These learnings have resulted in the approval of daratumumab in combination with lenalidomide, pomalidomide, bortezomib, or carfilzomib, plus dexamethasone.7 Isatuximab is approved in combination with either pomalidomide or carfilzomib plus dexamethasone, for the treatment of RRMM.7 Further to this, teclistamab and talquetamab (T-cell engagers that target MM antigens and the CD3 subunit of T-cell receptors) are being investigated in combination with daratumumab for RRMM in a phase Ib trial (TRIMM-2). Bi- and tri-specific T-cell engagers under investigation include ISB-1342, SAR442257, elranatamab, and cevostamab in combinations for the treatment of MM.1
Alternative strategies to overcome resistance1
Inhibiting molecules overexpressed in MM or those involved in extracellular adenosine production is another way to overcome resistance to CD38 antibodies. For example, CD39, involved in adenosine production, upregulates endothelial cells and CD73 expression, making it a target to overcome resistance to current MM therapies. A phase I trial of ORIC-533 (CD73 inhibitor) in patients with RRMM is underway.
Targeting both CD39 and CD73 may synergistically lower the levels of adenosine production. A phase I trial is currently investigating IPH5201 (CD39 antibody) in combination with durvalumab (anti-PD-L1) and/or oleclumab (CD73 mAb) in advanced solid tumors.
All-trans retinoic acid, or ATRA, has been proposed to upregulate CD38, enhancing the efficacy of anti-CD38 antibodies. However, ATRA combined with daratumumab has not shown marked benefit in a phase I/II trial in patients with RRMM.1
Conclusion
Optimizing treatments for patients with MM requires a comprehensive understanding of CD38 resistance mechanisms and ways to overcome them. The ongoing efforts to overcome CD38 resistance have resulted in the development of novel strategies, including combination regimens aimed at restoring sensitivity and improving patient outcomes.
This educational resource is independently supported by Sanofi. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



