All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
OCEAN subgroup analysis: Melflufen in patients refractory to prior alkylators
Melphalan flufenamide (melflufen), a first-in-class alkylating peptide drug conjugate, plus dexamethasone was recently approved in Europe for the treatment of multiple myeloma after ≥3 prior lines of treatment. Approval was based on results from the phase II HORIZON (NCT02963493) and phase III OCEAN trial (NCT03151811). An increasing number of patients with multiple prior lines of therapy are becoming exposed to, or refractory to alkylators which may alter the efficacy of melflufen.
In response to this, Schjesvold et al.1 published a post hoc analysis in European Journal of Haematology investigating the effects of refractoriness to standard dose prior alkylators on the effectiveness of melflufen + dexamethasone in a subset of patients enrolled in the OCEAN trial. We summarize the results below.
Study design1
- Patients randomized 1:1 to receive melflufen + dexamethasone or pomalidomide + dexamethasone.
- The primary endpoint was progression-free survival.
Key findings1
- N = 153
- n = 78 (melflufen + dexamethasone)
- n = 75 (pomalidomide + dexamethasone)
- Response rates in patients refractory to prior alkylators by treatment arm, prior autologous stem cell transplant status and type of alkylator received are listed in Figure 1.
Figure 1. Response rates in patients refractory to prior alkylators and treated with melflufen or pomalidomide*
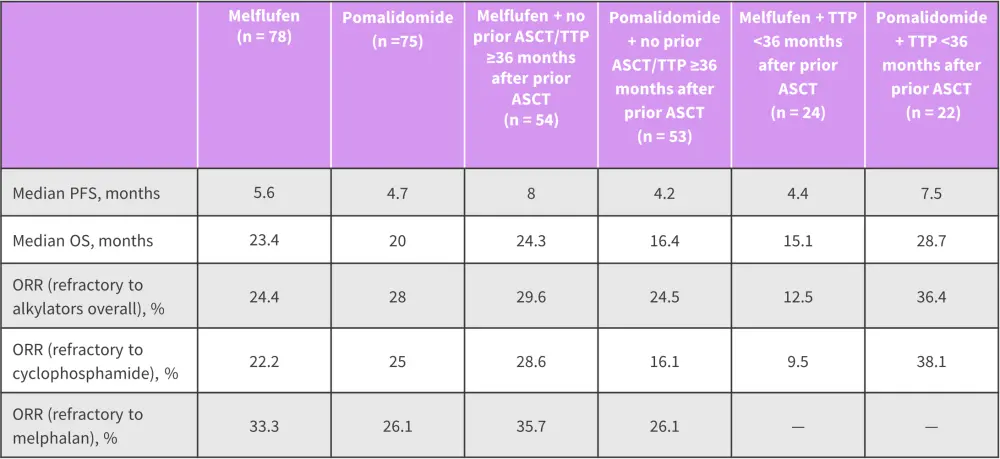
ASCT, allogeneic stem cell transplant; OS, overall survival; ORR, overall response rate; PFS, progression-free survival; TTP, time to progression.
*Adapted from Schjesvold, et al.1
- Treatment-emergent adverse event frequency was comparable between melflufen and pomalidomide treatment arms (99% vs 97%, respectively).
- Patients treated with melflufen experienced a higher frequency of dose modifications and dose reductions vs pomalidomide (76% vs 67% and 47% vs 14%, respectively)
- Patients treated with melflufen experienced higher rates of Grade 3/4 thrombocytopenia (73% vs 14%), neutropenia (65% vs 55%) and leukopenia (14% vs 3%) compared to pomalidomide.
|
Key learnings |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


