All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
EMA and FDA licensing of melphalan flufenamide for RRMM
Melphalan flufenamide (melflufen) is a peptide–drug conjugate, with alkylating properties affected by myeloma cell aminopeptidases.1 The drug was first given accelerated approval by the U.S. Food and Drug Administration (FDA) in February 2021, to be given in combination with dexamethasone for patients with relapsed/refractory multiple myeloma (RRMM).
The approval was based on the results of the phase II HORIZON trial (NCT02963493) in heavily pretreated patients with RRMM, who were refractory to one proteasome inhibitor (PI), one immunomodulatory agent (IMiD), one CD38-directed monoclonal antibody, and had been treated with at least four lines of therapy.2 The overall response rate was 29%, with responses lasting for a median of 5.5 months, and the overall survival (OS) was 11.6 months.2
In July 2021, the FDA called for a partial hold on all clinical studies involving melflufen, due to the increased risk of death observed in a prespecified patient analysis from the phase III OCEAN trial (NCT03151811).3 Subsequent to this, on October 22, 2021, melflufen was withdrawn from the US market.4,5 The decision was based on early analysis of the OCEAN trial, which demonstrated a hazard ratio (HR) of 1.104 in the intention-to-treat population, signifying poorer OS compared with the approved regimen pomalidomide plus dexamethasone.4–6 The drug was withdrawn following a request for voluntary withdrawal from the FDA, agreed to by the manufacturer.
After further subanalysis of the OCEAN trial, the voluntary withdrawal of melflufen was rescinded by the manufacturer in January 2022, with suggestion that autologous stem cell transplantation heterogeneity among patients had confounded the analysis.7,8 Patients with RRMM who had progressed within 3 years of transplant and treated with melflufen had an OS of 15.7 months compared with 28.7 months in the pomalidomide arm, with a respective OS of 23.6 months and 19.8 months in patients treated ≥3 years after progression.8
The initial analysis of progression-free survival (PFS) in the OCEAN study submitted to the FDA indicated that the primary objective had not been met. However, a later analysis showed that the OCEAN trial met the primary end point of improvement in PFS (HR, 0.792; 95% confidence interval, 0.640-0.979; p = 0.0311). Despite the additional data provided by the sponsor, the FDA Oncologic Drug Advisory Committee voted on September 22, 2022, that melflufen was not favorable for the treatment of patients with RRMM as currently indicated due to efficacy and safety concerns. Further details on the post hoc analyses and the FDA response can be found here.
As shown in Figure 1, melflufen efficacy and safety were evaluated in other trials, including the LIGHTHOUSE trial (NCT04649060). The LIGHTHOUSE phase III clinical trial compared melflufen plus subcutaneous daratumumab with dexamethasone against subcutaneous daratumumab with supportive dexamethasone.10 The study population was again patients refractory to at least one PI and IMiD or who had received at least three prior lines of therapy, including a PI and an IMiD.10 The open-label trial was originally designed as a similar confirmatory study to the OCEAN trial. After commencing in December 2020 with a plan to recruit 240 patients, the trial was halted as part of the FDA holds on clinical trials involving melphalan in July 2021, and later terminated in February 2022 with only 27 patients in each arm.10 With incomplete recruitment, the observed efficacy and safety of subcutaneous daratumumab in the control arm was in keeping with drug labels, but showed superior PFS with a HR of 0.062 (p = 0.0005) and superior OS with a HR of 0.00 (p = 0.037) in patients who had progressed within 3 years of autologous stem cell transplantation.10
Figure 1. Historical and ongoing clinical trials involving melflufen*
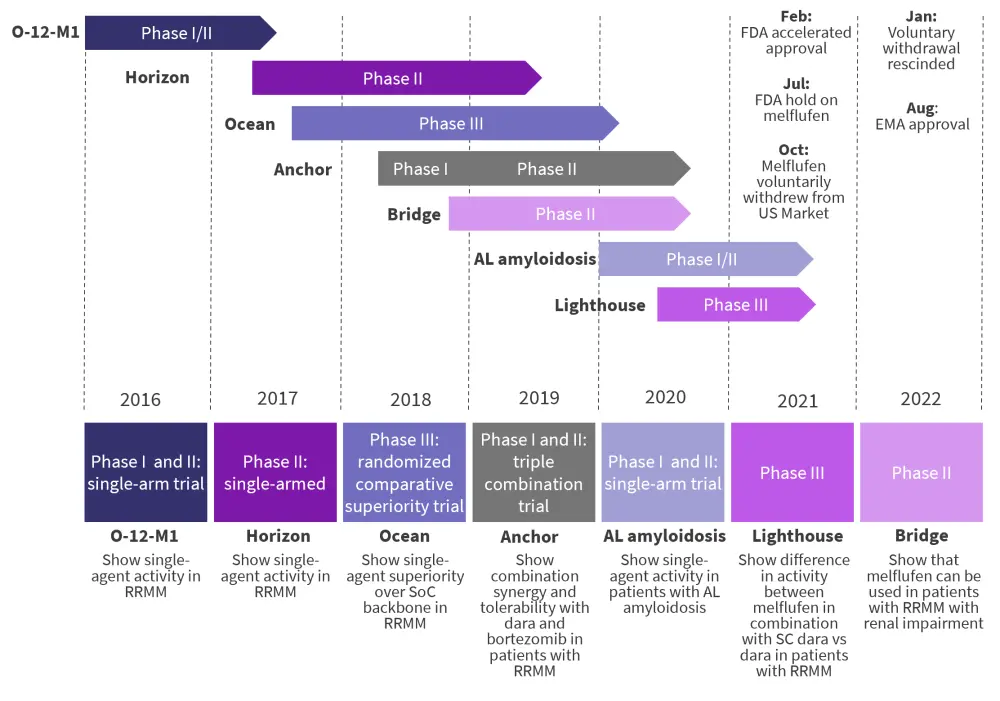
Dara, daratumumab; EMA, European Medicines Agency; FDA Food and Drug Administration; RRMM, relapsed/refractory multiple myeloma; SC, subcutaneous; SoC, standard of care.
*Adapted from Recipharm.11
In August 2022, melflufen was approved by the European Commission (again in combination with dexamethasone), for the treatment of adult patients with RRMM who have received at least three prior lines of therapies, including at least one PI, at least one IMiD, and one anti-CD38 monoclonal antibody, who have demonstrated disease progression on or after the last therapy.11 The approval was based on the HORIZON trial and supported by data from the OCEAN trial as discussed,12,13 and includes the specification that time to progression must be ≥3 years in patients who have undergone ASCT.14 In 2002, the manufacturer indicated its intention to apply to the European Medicines Agency (EMA) for consideration of melflufen in earlier lines of therapy for MM.15 Melfulfen is currently being actively marketed in Austria and Germany.16
Conclusion
Melflufen is an example of the challenges that can be faced by both regulatory agencies and drug companies when balancing attempts to bring promising new agents to patients as quickly as possible while trying to prevent increases in mortality and morbidity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



