All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
MIDAS: MRD-driven strategy after Isa-KRd induction in newly diagnosed ASCT-eligible MM
Featured:
The Multiple Myeloma Hub spoke with Paul Richardson, Dana-Farber Cancer Institute, Boston, Massachusetts, US. We asked about the clinical implications of findings from the phase III MIDAS trial (NCT04934475), which evaluated a measurable residual disease (MRD)-driven consolidation and maintenance strategy after induction with isatuximab + carfilzomib + lenalidomide + dexamethasone (Isa-KRd) in patients aged <66 years with newly diagnosed (ND), autologous stem-cell transplantation (ASCT)-eligible multiple myeloma (MM) (N = 791).1,2
MIDAS: MRD-driven strategy after Isa-KRd induction in ND ASCT-eligible MM
MIDAS: MRD-driven strategy after Isa-KRd induction in ND ASCT-eligible MM
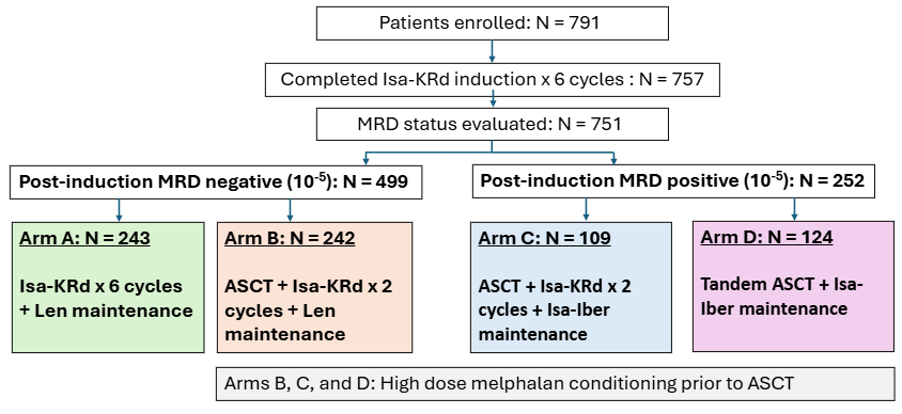
The primary end point of the MIDAS trial was measurable residual disease (MRD)-negative status at 10−6 sensitivity before maintenance therapy. An additional aim was to evaluate the benefit of high-dose melphalan with ASCT (the current standard care) compared with Isa-KRd alone in patients who were MRD-negative at 10−5 sensitivity post induction.1,2
MIDAS study design1,2
During this interview, Richardson discussed findings from the MIDAS trial, published by Perrot et al. in Blood and NEJM, and presented at the 2025 American Society of Clinical Oncology Annual Meeting (2025 ASCO Annual Meeting).
Key learnings
- Excellent CD34 stem cell yields and high response rates were achieved.1
- The median stem cell yield was 7 × 106/kg; 94% of patients were able to proceed with tandem transplant.
- 95% best overall response rate (ORR).
- 91% very good partial response rate (VGPR).
- Safety was manageable, with no new safety signals observed. However, cardiac events (likely related to carfilzomib) occurred in a small number of patients.1
- Seven patients experienced disease progression; five died due to disease progression (n = 1), cardiac events (n = 2), or other causes (n = 2).
- The most common Grade 3/4 adverse events (AEs) were neutropenia (25%), thrombocytopenia (5%), and infections (7%).
- Only 13% of patients reported any grade peripheral neuropathy.
- At the end of induction, MRD negativity was high; 63% at 10⁻⁵, and 47% at 10⁻⁶.
MRD-negative patients 2,3
- An additional six cycles of Isa-KRD (Arm A) vs single transplant + two additional cycles of Isa-KRd (Arm B) yielded similar MRD-negative rates (84% vs 86%), with no significant statistical difference (OR 1.17 [95% CI, 0.64–2.76]; p = 0.64).
- This suggests that transplant may be safely deferred in standard-risk, MRD-negative patients.
MRD-positive patients (considered higher risk, irrespective of stratification) 2,3
- Single transplant + Isa-KRD (Arm C) vs tandem transplant (Arm D) showed 40% vs 32% MRD negativity at 10⁻⁶, with no statistically significant difference (OR 0.73 [95% CI, 0.42–1.35]; p = 0.31).
- Tandem transplant did not outperform single transplant, challenging traditional assumptions.
Cytogenetic subgroups
- t(11;14): patients converted to MRD negativity more slowly but did have worse outcomes if they remained MRD positive.
- t(4;14): patients appeared to derive greater benefit from transplant, consistent with prior studies.
Overall findings and clinical implications 1–3
- These findings suggest the need for a tailored approach to transplant, as some patients (e.g. standard risk, MRD-negative) may benefit from a transplant-sparing approach.
- Supports risk-adapted, MRD-guided treatment.
- Quadruplet therapy with CD38 antibody + proteasome inhibitor (PI) + IMiD + steroid has been transformative for frontline myeloma treatment.
- There is the potential for future integration of immune therapies (CAR-T, bispecifics) and next-generation agents (e.g. iberdomide).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Paul Richardson
Paul Richardson

.jpeg&w=3840&q=75)