All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Isatuximab-based quadruplet induction achieves high MRD-negative rates in patients with newly diagnosed MM
The standard of care treatment for patients newly diagnosed with multiple myeloma (MM) and eligible for autologous stem cell transplantation (auto-SCT) has traditionally been lenalidomide, bortezomib, and dexamethasone.1 While the value of adding a CD38 monoclonal antibody to this combination treatment has shown potential, this remains to be confirmed in a phase III randomized trial.1
In response to this, Goldschmidt et al.1 recently published results in Lancet Haematology from the first part of the phase III GMMG-HD7 trial (NCT03617731), investigating the CD38 monoclonal antibody isatuximab, together with lenalidomide, bortezomib, and dexamethasone (RVd) compared with RVd alone for the treatment of patients with newly diagnosed MM (NDMM) who are eligible for auto-SCT. Isatuximab is also being evaluated in the GMMG-CONCEPT trial (NCT03104842) in combination with carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in patients with high-risk NDMM; Weisel2 presented the interim analysis results at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition.2 We summarize these recent results in the article below.
GMMG-HD7: Phase III trial of Isa-RVd vs RVd in transplant-eligible NDMM1
Study design
The open-label, multicenter, randomized phase III trial took place across 67 centers in Germany.
The eligibility criteria were as follows:
- Aged 18–70 years
- Confirmed diagnosis of untreated MM and measurable disease
- Eligible for induction therapy, high-dose melphalan, and autologous hematopoietic stem cell transplant
- World Health Organization performance status 0–2
- Platelet count ≥75 × 109 platelets/L
- Hemoglobin concentration >8 g/dL
- Neutrophil count ≥1 × 109 cells/L
- Serum calcium concentration <3.5 mmol/L
The exclusion criteria were as follows:
- Severe renal impairment (creatinine clearance <30 ml/min)
- Peripheral neuropathy or Grade 2 neuropathic pain
- Plasma cell leukemia
- Systemic light chain amyloidosis
- Other active malignancies
Eligible patients were randomly assigned an induction treatment with isatuximab with the combination treatment (Isa-RVd) or RVd at a 1:1 ratio.
Each patient was administered three cycles of treatment, with 42 days assigned to each cycle. The full treatment protocol of part 1 is highlighted in Figure 1.
Figure 1. GMMG-HD7 part 1 treatment protocol and endpoints*
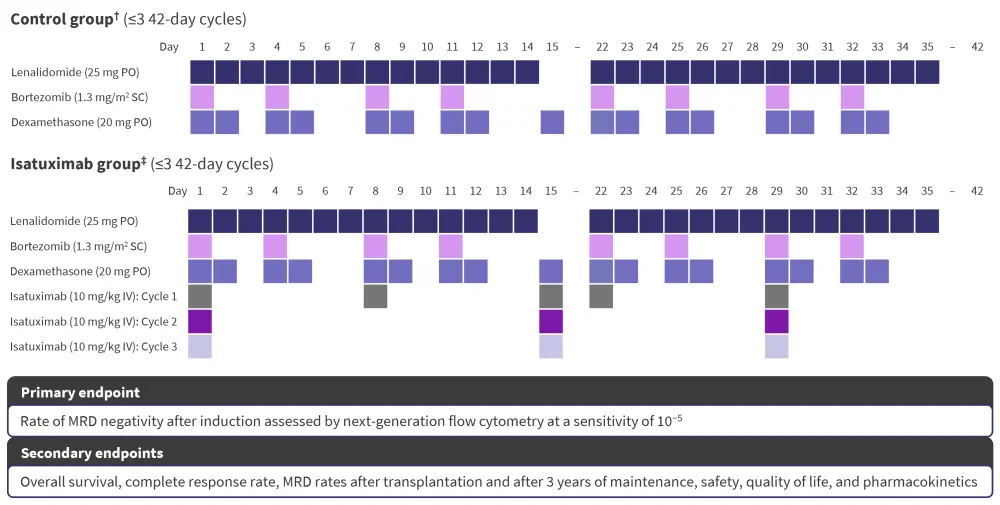
IV, intravenously; MRD, measurable residual disease; PO, orally; SC, subcutaneously.
*Data from Goldschmidt, et al.1
†Three cycles of induction therapy with lenalidomide, bortezomib, and dexamethasone.
‡Three cycles of induction therapy with isatuximab plus lenalidomide, bortezomib, and dexamethasone.
Efficacy
A total of 660 patients were included in the final study and were analyzed between October 2018 and September 2020 (Table 1).
Table 1. Baseline patient characteristics*
|
ISS, International Staging System; ISS-R, Revised International Staging System; WHO, World Health Organization. |
||
|
Characteristic, % (unless otherwise stated) |
Isatuximab group |
Control group |
|---|---|---|
|
Median age at randomization, years |
59 |
60 |
|
Sex |
|
|
|
Female |
38 |
37 |
|
Male |
62 |
63 |
|
Ethnicity, n |
|
|
|
White |
327 |
327 |
|
African |
1 |
1 |
|
Arabic |
1 |
0 |
|
Asian |
2 |
1 |
|
WHO performance status |
|
|
|
0 |
48 |
51 |
|
1 |
41 |
40 |
|
2 |
11 |
9 |
|
3 |
0 |
<1 |
|
ISS disease stage |
|
|
|
I |
37 |
45 |
|
II |
39 |
35 |
|
III |
24 |
20 |
|
High-risk cytogenetics† |
|
|
|
No |
77 |
71 |
|
Yes |
18 |
20 |
|
Unknown |
6 |
9 |
|
ISS-R disease stage |
|
|
|
I |
23 |
30 |
|
II |
66 |
56 |
|
III |
8 |
8 |
|
Not classified |
2 |
6 |
From the treatment group, 5% of patients discontinued treatment during the study, and 11% of patients discontinued from the control group. The rates of negative measurable residual disease (MRD) for both the treatment and control groups are shown in Figure 2, while the best response rates are shown in Figure 3.
Figure 2. Rates of negative MRD in the intention-to-treat population in the control and treatment groups*†
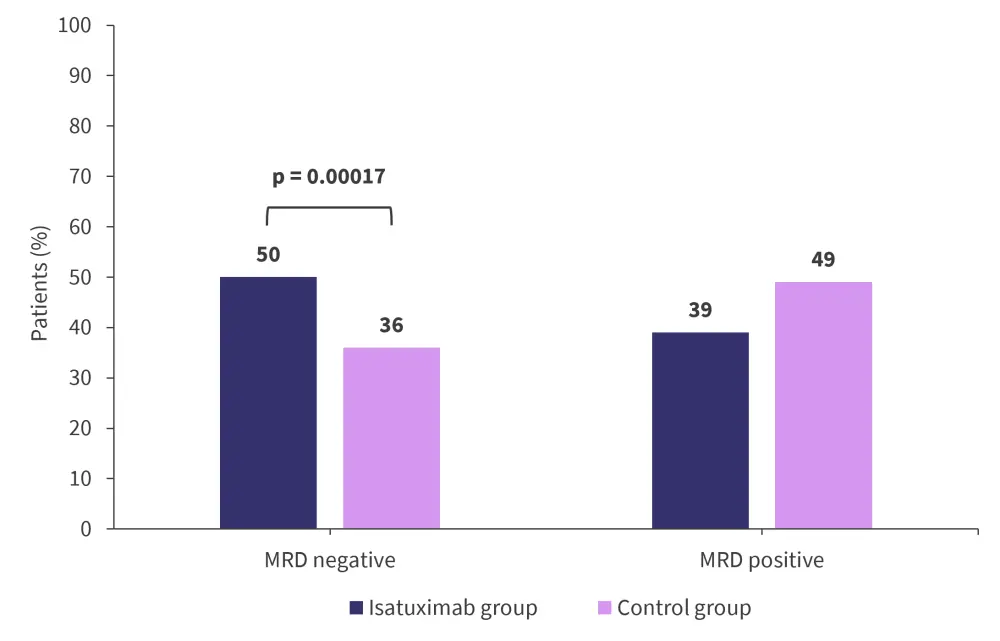
MRD, measurable residual disease.
*Adapted from Goldschmidt, et al.1
†MRD was assessed after a median of 10 days after induction treatment by next-generation flow cytometry at a 10−5 sensitivity.
Figure 3. Best response rates in the control and treatment groups*
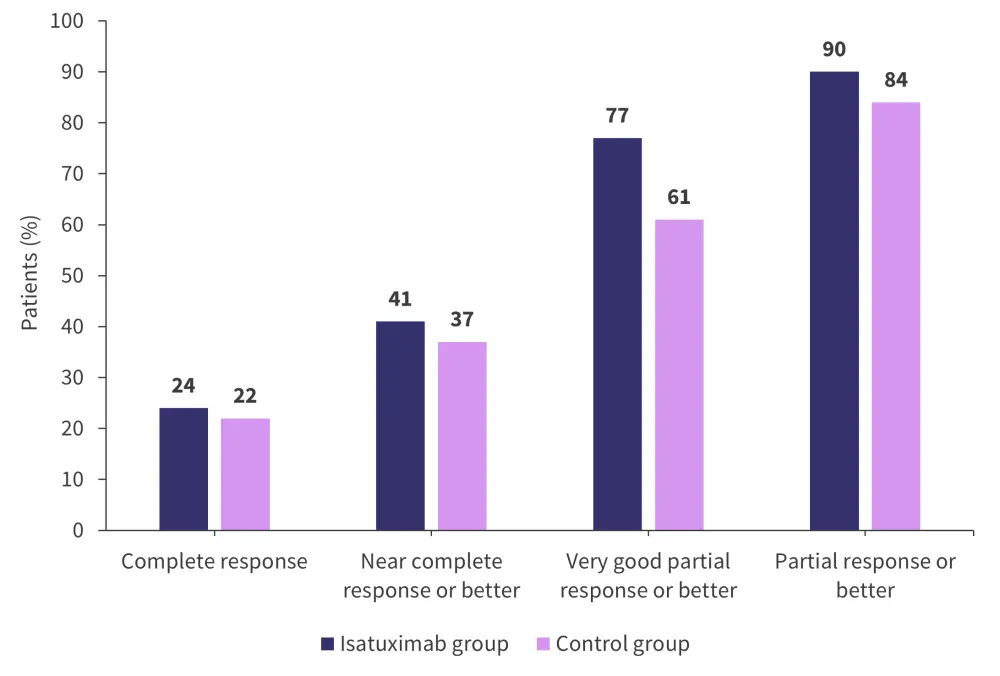
*Adapted from Goldschmidt, et al.1
A total of 18% of patients in the treatment group achieved both a complete response and negative MRD compared with 14% of patients in the control group (p = 0.17). A very good partial response with negative MRD was achieved by 47% of patients in the treatment group and 32% in the control group (p < 0.0001).
Safety
The percentage of patients who experienced ≥1 Grade 3 or 4 adverse event (AE) in the control and treatment groups was 61% and 63%, respectively. A total of 28% of patients from the treatment and control groups experienced ≥1 Grade 3 or 4 serious AE. The most common Grade 3 or 4 AEs are shown in Figure 4.
Figure 4. Most common Grade 3 or 4 adverse events*
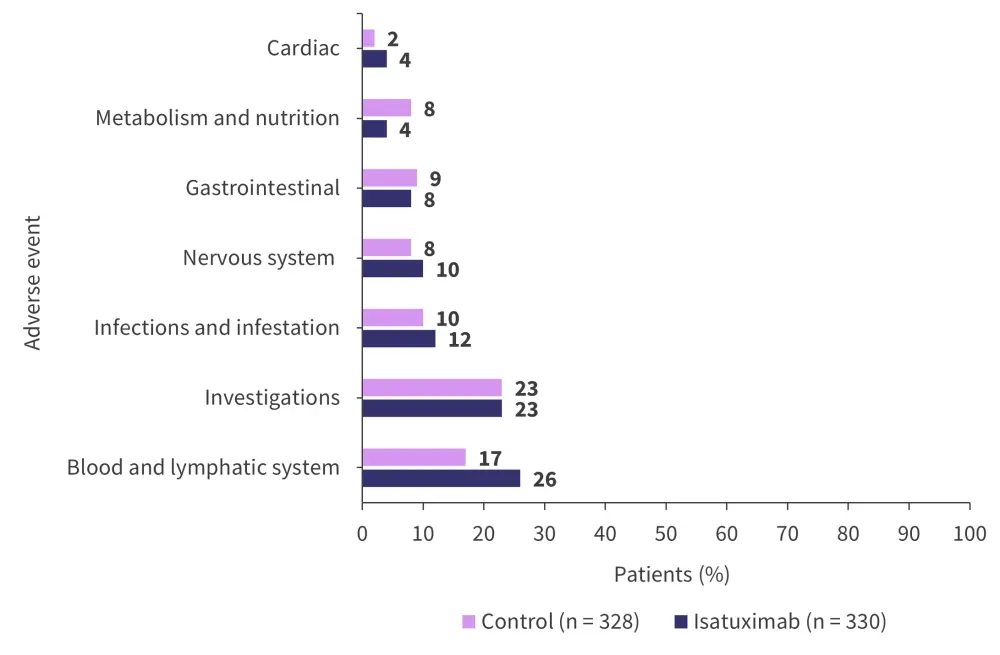
*Adapted from Goldschmidt, et al.1
Neutropenia of Grade 3 or 4 was experienced by 23% of patients treated with isatuximab and 7% in the control group. A total of 10% of patients from the control group experienced Grade 3 or 4 infections compared with 12% of patients treated with isatuximab. Grade 3 or 4 isatuximab infusion-related events were recorded in only 1% of patients.
A total of four deaths were recorded in the treatment group, of which one was considered treatment-related. In contrast, the control group recorded eight deaths, of which four were considered treatment-related. With post-induction, all the patients from the treatment group and 99% of patients from the control group underwent an autologous stem cell transplantation. A total of 99% of patients in both treatment groups achieved the minimum required stem cell harvest.
GMMG-CONCEPT: Phase II trial of Isa-KRd in high-risk NDMM2
The study design and baseline patient characteristics were previously reported in this Multiple Myeloma Hub article. High-risk disease was defined as including the presence of one or more of the following:
- Del17p
- t(4;14)
- t(14;16)
- 3 copies of 1q21
- International Staging System (ISS) II or III stage disease
Efficacy
The overall response rate for the patients eligible for transplant was 94.9% compared with 88.5% for patients who were ineligible for transplant. The MRD rates (assessed at a 10−5 sensitivity) for patients eligible for transplant and patients transplant ineligible are shown in Figure 5. The best responses through consolidation treatment are highlighted in Figure 6.
Figure 5. Rates of MRD after consolidation with Isa-KRd*
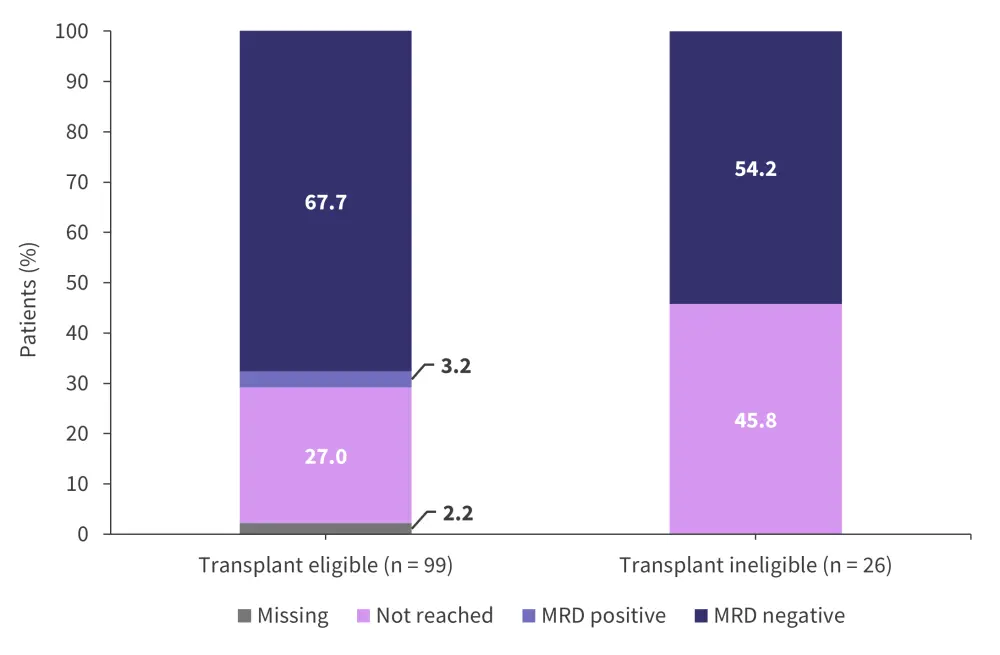
Isa-KRd, isatuximab + carfilzomib + lenalidomide + dexamethasone; MRD, measurable residual disease.
*Adapted from Weisel.2
Figure 6. Best responses through consolidation with Isa-KRd*
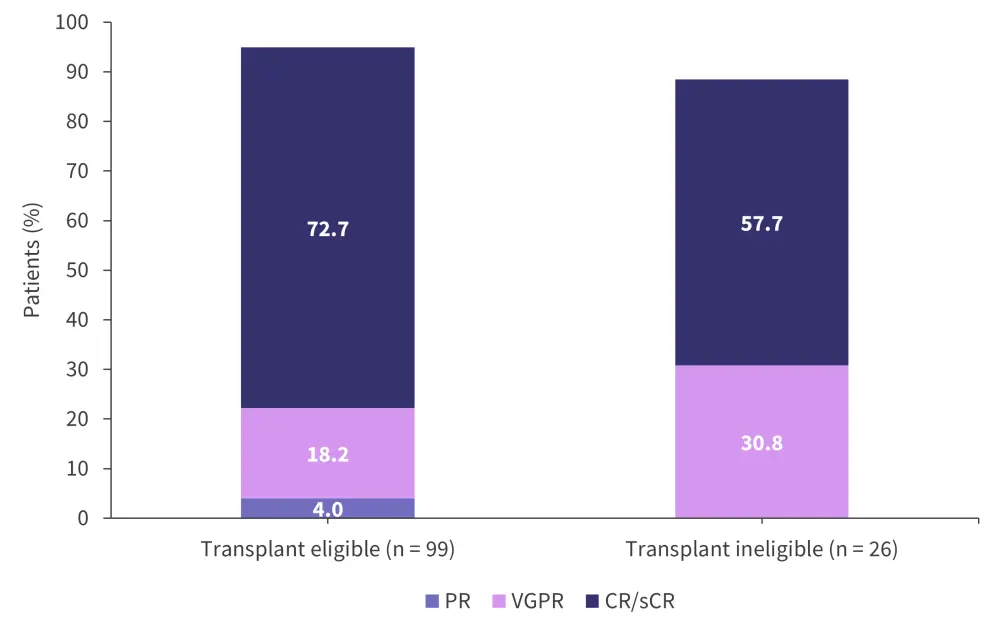
CR, complete response; Isa-KRd, isatuximab + carfilzomib + lenalidomide + dexamethasone; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Weisel.2
Safety
A total of 78.4% of patients eligible for transplant experienced ≥3 AEs of any grade compared with 72% of patients ineligible for transplant. The most common AEs of any grade for both treatment arms are shown in Figure 7.
Figure 7. Most common adverse events of any grade*
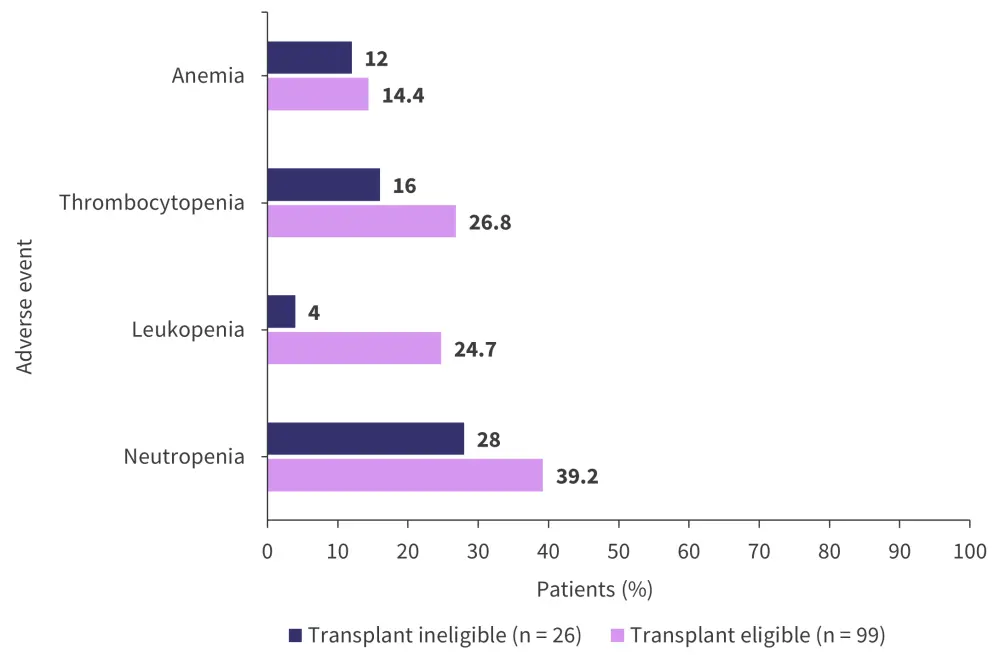
*Adapted from Weisel.2
Conclusion
The addition of isatuximab to the standard triplet combination of lenalidomide, bortezomib, and dexamethasone resulted in a statistically significant improvement in rates of negative MRD compared with the standard triplet combination alone. There was also no overall difference in toxicities between the two treatment groups. Limitations of the GMMG-HD7 study include a short follow-up time, together with a lack of progression-free survival data.
In comparison, the addition of Isatuximab to carfilzomib, lenalidomide, and dexamethasone is being explored in a selected high-risk patient cohort and showed high rates of deep responses that improve over time. A total of 74% of patients in the trial reached the primary endpoint of the GMMG-CONCEPT trial. The combination was also well tolerated, with a manageable safety profile and low rates of peripheral neuropathy.
Longer follow-up from these trials will help elucidate the future role of isatuximab-based quadruplet combinations in frontline treatment.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

