All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Interim analysis on the Isa-KRd quadruplet regimen for patients with high-risk NDMM
Featured:
The addition of anti-CD38 antibodies within induction therapies has produced markedly improved outcomes in response rates and minimal residual disease (MRD) negativity in patients with newly diagnosed multiple myeloma (NDMM). However, data are lacking for the high-risk subset of patients who have been historically unrepresented in large trials for multiple myeloma. Thus, there is an unmet need for a more defined frontline treatment that overcomes the poor prognosis of this subgroup of patients.
At the 26th Congress of the European Hematology Association (EHA2021), Katja Weisel, University Medical Center Hamburg-Eppendorf, Hamburg, DE, presented an updated interim analysis of the ongoing GMMG-CONCEPT trial (NCT03104842).1 This phase II trial investigated a quadruple combination of the anti-CD38 antibody isatuximab, plus carfilzomib, lenalidomide, and dexamethasone (Isa-KRd), for patients with high-risk NDMM. Isatuximab was used as the anti-CD38 therapy, owing to previous evidence of its antitumor activity, multiple modes of action, and unique clinical profile. Carfilzomib was chosen over bortezomib as the proteasome inhibitor, because of its high efficacy in the frontline setting and superiority when used at relapse.1
Methods
The GMMG-CONCEPT trial is a phase II, multicenter study investigating Isa-KRd in the following treatment arms:
- Arm A: Transplant-eligible patients (n = 117), who received six cycles of Isa-KRd prior to autologous stem cell transplantation (auto-SCT), followed by four cycles of Isa-KRd consolidation, and finally, Isa-KR maintenance.
- Arm B: Transplant-ineligible patients (n = 36), who received an extra two cycles of Isa-KRd induction (eight cycles in total) followed by four cycles of Isa-KRd consolidation, and finally, Isa-KR maintenance.
The trial recruited 153 patients, and will reopen to enroll further patients (n = 93) in Arm A. In this interim analysis, the first 50 patients receiving the study induction therapy were included. The dosing schedule for induction cycles is summarized in Figure 1.
Figure 1. Isa-KRd dosing schedule for Cycle 1 (28 days) and Cycles 2−6 (28 days) of induction therapy*
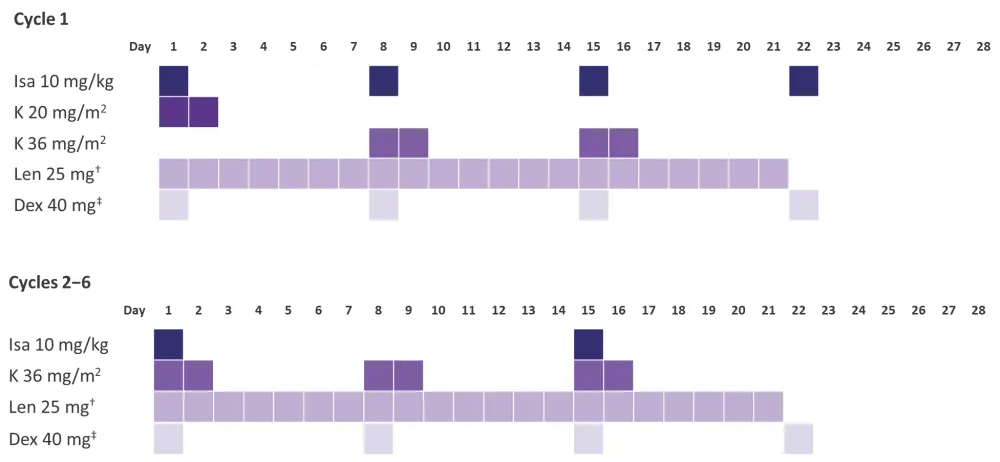
Dex, dexamethasone; Isa, Isatuximab; K, carfilzomib; Len, lenalidomide; Isa-KRd, isatuximab plus carfilzomib, lenalidomide, and dexamethasone.
*Adapted from Leypoldt et al.1
†Dose adaptation of lenalidomide according to renal function.
‡20 mg in patients ≥75 years.
Endpoints
- Primary endpoint: MRD negativity following consolidation measured by next-generation flow with a sensitivity of 10−5.
- Secondary endpoint: Progression-free survival (PFS).
- Tertiary endpoints: Overall response rate (ORR), duration of MRD negativity, overall survival (OS), assessment of the quality of life.
- Safety data included Grade 3/4 hematologic and nonhematologic adverse events (AEs).
Eligibility criteria
- Patients with NDMM and high-risk disease; including the presence of one or more of the following:
- Del17p
- t(4;14)
- t(14;16)
- > 3 copies 1q21
- International Staging System (ISS) II or III stage disease
- No more than one cycle (4 weeks) of anti-myeloma medication prior to study treatment.
- Patients must have had adequate organ function.
Results
Patient characteristics for the interim analysis cohort (n = 50) are summarized in Table 1.
Table 1. Patient characteristics*
|
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System. |
|
|
Characteristic |
N = 50 |
|---|---|
|
Median age, years (range) |
58 (42−82) |
|
Arm A |
58 (42−69) |
|
Arm B |
77 (72−82) |
|
Female, % |
58 |
|
ECOG performance status, % |
|
|
0 |
42 |
|
1 |
46 |
|
2 |
12 |
|
ISS disease stage, % |
|
|
II |
56 |
|
III |
44 |
|
High-risk cytogenetics, % |
|
|
Del17p |
52 |
|
t(4;14) |
38 |
|
t(14;16) |
12 |
|
>3 copies 1q21 |
42 |
|
Any two high-risk aberrations |
26 |
Primary endpoint
- MRD assessment was recommended in patients achieving a very good partial response (VGPR).
- For transplant-eligible patients (n = 33), 20 (60.6%) were MRD-negative during induction, while 11 (33.3%) patients were MRD-positive. Two patients were not assessable.
- Data were not available yet for the MRD negativity status following induction, but the first cohort results (N = 153) are expected to be reported in 2022.
Secondary endpoints
- After a median follow-up of 24.9 months, a median PFS was not reached:
- 12-month PFS rate was 79.6%
- 24-month PFS rate was 75.5%
- 40/50 (80%) of patients were relapse-free after 1 year.
- Analysis of all 50 evaluable patients following six induction cycles revealed an ORR of 100%.
- 90% of patients achieved at least a VGPR, and 46% achieved a complete remission/stringent complete remission.
- 41/46 (89.1%) of transplant-eligible patients achieved ≥VGPR, while all four transplant-ineligible patients achieved a VGPR.
Safety
- No new safety signals were observed. Grade 3−4 hematologic and nonhematologic toxicities are summarized in Table 2.
Table 2. Summary of Grade 3−4 treatment-emergent adverse events (TEAEs)*
|
TEAE, treatment-emergent adverse event. |
|
|
N = 50 |
Grade 3−4 TEAE |
|---|---|
|
Hematologic, % |
|
|
Leukopenia |
26 |
|
Neutropenia |
34 |
|
Lymphopenia |
28 |
|
Anemia |
10 |
|
Thrombocytopenia |
14 |
|
Febrile neutropenia |
4 |
|
Nonhematologic, % |
|
|
Peripheral sensory neuropathy |
2 |
|
Hypertension |
12 |
|
Cardiac failure |
4 |
- The authors highlighted a low incidence of peripheral sensory neuropathy.
- For Grade 1/2 TEAEs associated with anti-CD38 administration, 32% of patients had an infusion reaction, 18% reported upper respiratory tract infection, and 16% reported rash.
- Four cases of COVID-19 were reported, resulting in one death.
- 20/21 (95.2%) of patients discontinuing treatment were in the transplant-eligible cohort. Only one of these patients discontinued treatment due to unacceptable toxicity.
- Five deaths were reported: four were attributable to infections (pulmonal sepsis, influenza A, pneumonia, and neutropenic sepsis), and one died of an unknown cause.
Conclusions
Overall, the updated interim analysis of the GMMG-CONCEPT trial demonstrated deep and durable responses with an Isa-KRd quadruple regimen for patients with high-risk NDMM. Encouragingly, there was a high PFS rate at 12 and 24 months (79.6% and 75.5%, respectively), and a significant number of patients achieved MRD negativity during induction, which is associated with improved long-term prognosis. Although response appeared to be irrespective of transplant eligibility, a greater number of transplant-ineligible patients is required for a more substantial consensus. A favorable safety profile was maintained; however, the investigators highlighted the need to monitor infections continuously alongside the treatment.
For further information on this interim analysis, watch below the Multiple Myeloma Hub interview with Katja Weisel.
Can Isa-KRd achieve deep responses in high-risk NDMM, regardless of transplant eligibility?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Katja Weisel
Katja Weisel