All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
In light of the encouraging outcomes observed in the ICARIA-MM study (NCT02990338), evaluating isatuximab in combination with pomalidomide and dexamethasone (Isa-Pd) for the treatment of adult patients with relapsed/refractory multiple myeloma (RRMM), Isa-Pd has been approved in a number of countries. The triplet regimen is indicated for the treatment of adult patients with RRMM following treatment with ≥2 prior therapies, including lenalidomide and a proteasome inhibitor.
A recent subgroup analysis of the pivotal ICARIA-MM study sought to determine whether frailty influences the clinical outcomes and safety of Isa-Pd in patients with RRMM. The study utilized a simplified frailty score that considered patient age, modified Charlson Comorbidity Index (CCI), and Eastern Cooperative Oncology Group (ECOG) performance score. In a letter to the editor, Fredrik Schjesvold and colleagues1 provided the results from this subgroup analysis, and the Multiple Myeloma Hub is pleased to provide a summary.
Results
Patient disposition
- Of the 307 patients enrolled in the ICARIA-MM study
- 154 and 153 were assigned to the Isa-Pd and Pd arms, respectively;
- 28.0% were considered frail;
- 69.4% were considered fit/intermediate; and
- 2.6% were not included in this subgroup analysis due to inadequate medical history records.
- There was not a significant difference in the number of frail patients in the Isa-Pd (31.2%) vs Pd arms (24.8%; p = 0.2167).
Efficacy
- Although not significantly different, progression-free survival was superior in patients who received Isa-Pd vs Pd, irrespective of frailty status (Table 1), and outcomes from this subgroup analysis are comparable to those observed in the overall study population.
- Overall response rates were not significantly different between patients receiving Isa-Pd classed as frail vs fit/intermediate and superior in patients who received Isa-Pd vs Pd, irrespective of frailty status (Figure 1).
Table 1. Patient outcomes to Isa-Pd vs Pd by frailty status*
|
Isa, isatuximab; Pd, pomalidomide + dexamethasone; PFS, progression-free survival. |
||||
|
|
Frail |
Fit/intermediate |
||
|---|---|---|---|---|
|
Isa-Pd |
Pd |
Isa-Pd |
Pd |
|
|
PFS, months |
9.0 |
4.5 |
12.7 |
7.4 |
|
1-year OS, % |
66.9 |
58.8 |
75.0 |
64.5 |
Figure 1. ORRs to Isa-Pd vs Pd by frailty status*
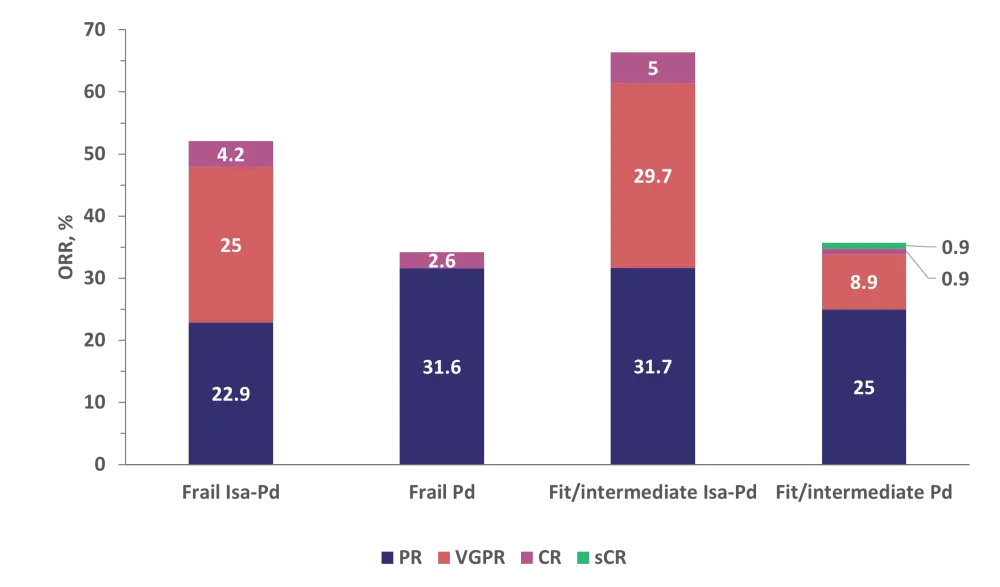
CR, complete response; Isa, isatuximab; ORR, overall response rate; Pd, pomalidomide + dexamethasone; PR; partial response; sCR, stringent complete response; VGPR, very good partial response.
*Data from Schjesvold, et al.1
Safety
- All patients classified as frail experienced treatment-emergent adverse events (TEAEs), irrespective of treatment arm.
- The most commonly observed non-hematologic TEAEs in frail and fit/intermediate patients were diarrhea and infusion reactions, respectively (Table 2).
- Rates of anemia and thrombocytopenia were comparable between patients in the Isa-Pd vs Pd arms irrespective of frailty, and Grade 4 neutropenia was elevated in patients who received Isa-Pd, again irrespective of frailty (Table 2).
Table 2. Safety overview of Isa-Pd vs Pd by frailty status*
|
IRR, infusion related reaction; Isa, isatuximab; Pd, pomalidomide plus dexamethasone; TEAE, treatment-emergent adverse event. |
||||
|
|
Frail |
Fit/intermediate |
||
|---|---|---|---|---|
|
|
Isa-Pd |
Pd |
Isa-Pd |
Pd |
|
TEAEs, % |
||||
|
Any grade |
100.0 |
100.0 |
100.0 |
98.2 |
|
Infections and infestations |
83.3 |
75.0 |
80.0 |
62.2 |
|
Diarrhea |
33.3 |
27.8 |
23.0 |
17.1 |
|
IRR |
31.3 |
0 |
40.0 |
0 |
|
Bronchitis |
27.1 |
11.1 |
22.0 |
8.1 |
|
Upper respiratory tract infection |
25.0 |
8.3 |
29.0 |
20.7 |
|
Dyspnea |
25.0 |
11.1 |
10.0 |
9.9 |
|
Pyrexia |
20.8 |
19.4 |
12.0 |
12.6 |
|
Hematologic abnormalities, % |
||||
|
Neutropenia |
|
|
|
|
|
Grade 4 |
68.8 |
31.4 |
58.0 |
31.8 |
|
Febrile neutropenia |
12.5 |
0 |
12.0 |
2.7 |
|
Other events |
||||
|
Death, % |
10.4 |
11.1 |
6.0 |
9.0 |
|
Treatment discontinuation*, % |
8.3 |
16.7 |
7.0 |
11.7 |
|
Time to treatment discontinuation, months |
8.7 |
4.9 |
9.4 |
5.3 |
Conclusion
In summary, frail patients with RRMM treated with Isa-Pd demonstrate similar clinical responses to fit/intermediate counterparts. Furthermore, the safety profile of the triplet regimen in frail patients was comparable to the overall study populations, and Isa-Pd discontinuation rates were lower in patients classed as frail vs fit/intermediate. Of note, this was not a prespecified analysis of the ICARIA-MM trial, but it indicates that it is feasible to treat frail patients with RRMM with a triplet regimen, which is encouraging in this difficult-to-treat population.
For more information on the considerations when treating frail patients with MM, watch the video with Sonja Zweegman below.
3 things you should consider when treating frail patients
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Sonja Zweegman
Sonja Zweegman