All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Improving access to CAR T-cell therapy for eligible patients with MM
Do you know... Earlier-line implementation of CAR T-cell therapy may be beneficial for patients due to which of the following reasons?
During the Multiple Myeloma Hub Steering Committee Meeting in November 2025, key opinion leaders met to discuss improving access to chimeric antigen receptor (CAR) T-cell therapy for eligible patients with multiple myeloma. The meeting opened with a presentation by Sagar Lonial and featured a discussion including Morie Gertz, Elena Zamagni, Meral Beksaç, and Sonia Zweegmann.
During his presentation, Lonial provided an overview of approved and investigational CAR T-cell therapies for multiple myeloma, the CAR T-cell therapy treatment process (Figure 1), and multi-step treatment pathway. He explored barriers to treatment with CAR T-cell therapy (Figure 2), racial disparities in access to CAR T-cell therapy, and manufacturing and attrition considerations. He discussed potential strategies for improving access to CAR T-cell therapy for eligible patients, exploring patient selection and referral, optimizing the pre-CAR-T infusion process, use of CAR T-cell therapy in earlier lines of therapy, accelerated manufacturing, and allogeneic CAR T-cell therapies.
Improving access to CAR T-cell therapy for eligible patients with MM
Improving access to CAR T-cell therapy for eligible patients with MM
Figure 1. Overview of the CAR T-cell therapy treatment process*
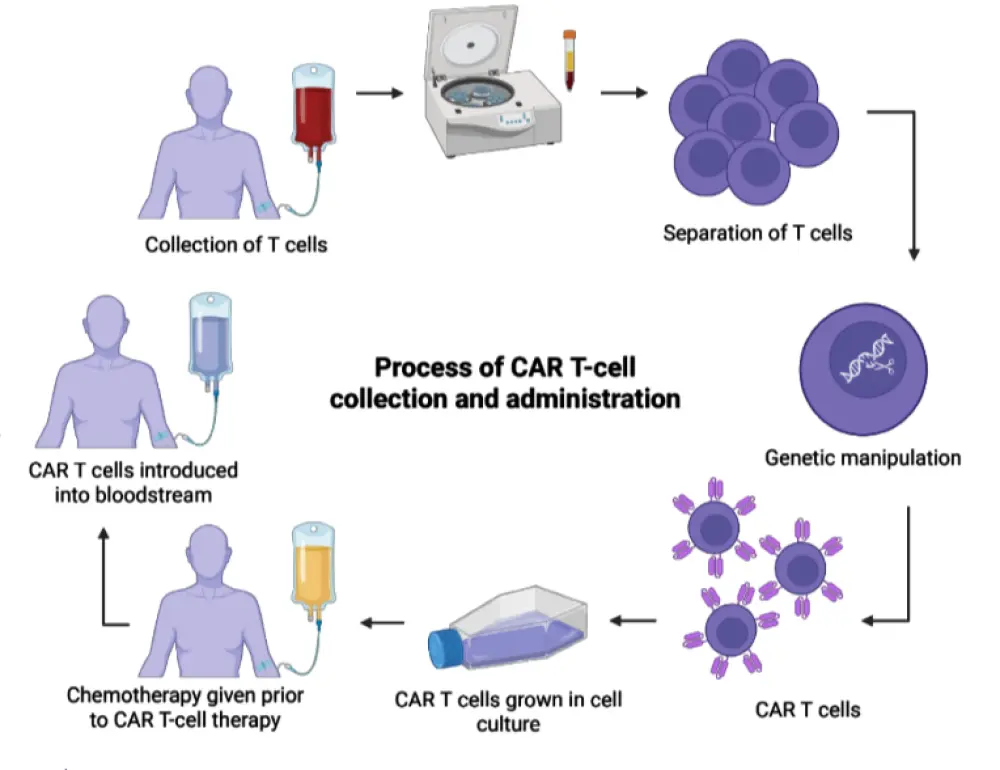
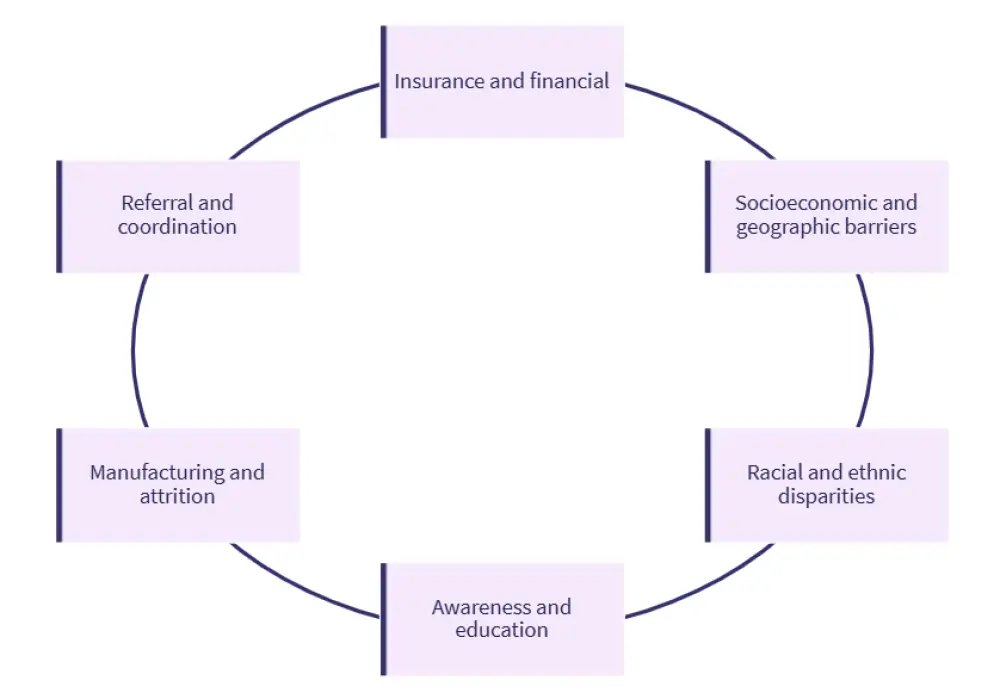
Figure 2. Barriers to CAR T-cell therapy*
Key points
- CAR T-cell therapy is a complex, multi-step process requiring close collaboration between referring clinicians, treatment centers, and manufacturers, with timing and logistics strongly influencing feasibility and outcomes.1,3
- Major barriers to CAR T-cell therapy access include patient identification, insurance limitations, financial strain, socioeconomic and geographic challenges, care coordination difficulties, manufacturing delays, attrition, racial and ethnic disparities, and limited healthcare professional and patient awareness.3,4
- Geographic distance and travel requirements significantly affect access, with temporary relocation often required, and travel needed for T-cell collection, lymphodepletion, infusion, and early post-infusion monitoring.3
- Challenges in the United States include low referral rates, complex insurance authorization, and substantial non-medical costs (lodging, meals, travel), which may influence treatment choices even when coverage is adequate.4
- European access varies widely, with some countries lacking reimbursement and others using national tumor boards to promote standardized and equitable referral.
- Early referral is critical; many centers rely on community oncologists to identify candidates before deterioration limits CAR T-cell therapy eligibility.
- Earlier-line implementation, especially for patients with functional high-risk disease or relapse within 4 years, may improve outcomes, reduce attrition, and allow patients to be treated when their performance status is better.1
- Bridging therapy remains essential, as high disease burden at infusion worsens outcomes.3
- Allogeneic CAR T-cell therapies may enable faster treatment by avoiding individualized manufacturing, though concerns remain about their persistence and durability in myeloma.1
- Post-CAR T-cell therapy supportive care and rural follow-up logistics remain challenging, underscoring the need for strong communication between community and academic centers.3
- Referral recommendations from an expert roundtable to optimize access to CAR T-cell therapy include:3
- Consideration of referrals for CAR T-cell therapy at least one line of therapy prior to eligibility.
- Early conversations regarding referrals for historically underserved patients (e.g. older, rural, lower socioeconomic status, racial/ethnic minorities).
- Early conversations regarding referrals for patients with high-risk MM at diagnosis.
- Consideration of performance status, rate of disease progression, organ function, patient willingness, and logistical or supportive requirements.
This discussion topic is supported by Kite through Gilead Sciences Europe Ltd, who provided funding. All content was developed independently by the steering committee in collaboration with SES. Funders were allowed no influence on the content of the discussion.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Elena Zamagni
Elena Zamagni Morie Gertz
Morie Gertz Sagar Lonial
Sagar Lonial Meral Beksaç
Meral Beksaç

