All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
KarMMa-9: Idecabtagene vicleucel plus lenalidomide for the treatment of NDMM with suboptimal response to ASCT
Patients with newly diagnosed multiple myeloma (NDMM) who experience an incomplete response to autologous stem cell transplantation (ASCT) have poorer outcomes, with an increased risk of progression (38%) and an increased risk of death (41%) compared with patients who experience a complete response.1 KarMMa-9 (NCT06045806) is an ongoing phase III trial comparing the efficacy and safety of idecabtagene vicleucel (ide-cel) plus lenalidomide for maintenance vs lenalidomide alone in patients with NDMM who had a suboptimal response (partial response or very good partial response) to ASCT.
Here we summarize a poster presentation by Mateos, et al.1 from the 5th European Myeloma Network Meeting, 2024, on the rationale and study design of the phase III KarMMa-9 trial.1
Study design and key findings1
- KarMMa-9 is an ongoing multicenter, randomized trial, currently enrolling across 19 countries.
- Eligibility criteria include:
- Patients with NDMM
- Aged ≥18 years
- East Cooperative Oncology Group (ECOG) performance status of 0–2
- Received 4–6 cycles of induction therapy
- Single ASCT 80–120 days prior to study treatment
- ‘Partial response’ or ‘very good partial response’ post-ASCT at the time of consent
- No prior maintenance after ASCT
- The primary endpoint is progression-free survival (PFS) per the independent review committee (IRC).
- The key secondary endpoint is overall survival (OS) The other secondary endpoints include measurable residual disease-negative complete response rate, event-free survival, duration of response, complete response rate per IRC, time to progression per IRC, PFS2, time to next treatment, safety, health-related quality of life, and pharmacokinetics.
- Key safety outcomes include AE reporting.
- Patients will be randomly assigned to receive ide-cel plus lenalidomide or standard-of-care lenalidomide maintenance (Figure 1).
- Combining ide-cel with standard-of-care maintenance therapy is expected to improve clinical outcomes and extend PFS in patients with clinically high-risk NDMM.
- Randomization will be stratified, based on the stage of disease, induction therapy, and the degree of treatment response post-ASCT.
Figure 1. KarMMa-9 study design*
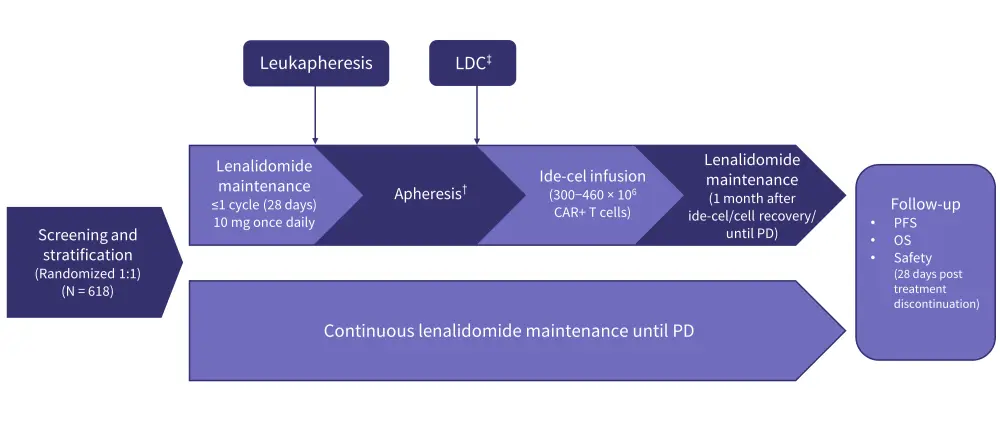
CAR, chimeric antigen receptor; ide-cel, idecabtagene vicleucel; LDC, lymphodepleting chemotherapy; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
*Adapted from Mateos, et al.1
†Apheresis to be performed within 14−42 days after last dose of R.
‡Fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 on Days -5, -4, and -3 prior to ide-cel infusion.
- The trial was initiated in October 2023 and is currently recruiting at 118 sites in 19 countries.
- Australia, Austria, Belgium, Canada, Czech Republic, Denmark, France, Germany, Greece, Israel, Italy, Japan, Korea, Norway, Poland, Romania, Spain, United Kingdom, and the USA
- A total of 618 eligible patients with NDMM have been enrolled so far.
|
Key learnings1 |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




