All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Dara-KRd and tandem ASCT for patients with high-risk NDMM: Results of the IFM-2018-04 trial
Patients with newly diagnosed multiple myeloma (NDMM) who are eligible for transplant and have high-risk cytogenetics traditionally have a poor prognosis.1 To improve this, different treatment combinations have been trialed with some success, such as carfilzomib, lenalidomide, and dexamethasone (KRd) in the FORTE trial and adding daratumumab (dara) to frontline therapy as in the CASSIOPEIA and GRIFFIN trials. In addition, tandem stem cell transplants have improved the outcome of these high-risk patients with NDMM.1
In the IFM-2018-04 trial, the quadruplet of dara-KRd with tandem transplant was assessed in patients with NDMM eligible for transplant; Touzeau1 presented the results of this trial at the European Hematology Association (EHA) 2022 Congress, which we are pleased to summarize here.
Study design
The dosing schedule and study design for the IFM2018-04 phase II trial (NCT03606577) are shown in Figure 1.
Inclusion criteria:
- Age, <66 years
- Patients with NDMM who are eligible for transplant
- High-risk fluorescence in situ hybridization: t(4;14), 17p del, and t(14;16)
- Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2
Endpoints:
- Primary:
- Feasibility: >70% of patients completed second transplant
- Secondary:
- Safety, overall response rate, progression-free survival (PFS), overall survival (OS) and stem cell collection
Figure 1. Study design*
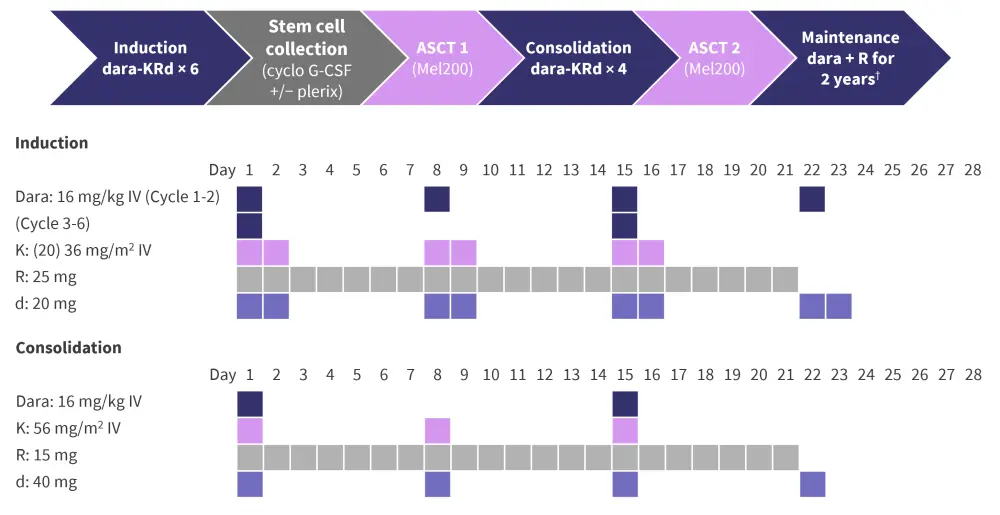
ASCT, autologous stem cell transplant; cyclo G-CSF, cyclophosphamide and granulocyte-colony stimulating factor; d, dexamethasone; dara, daratumumab; IV, intravenous; K, carfilzomib; Mel, melphalan; R, lenalidomide.
*Adapted from Touzeau.1
†Dara 16 mg/kg IV every 8 weeks + R 10 mg 21/28 days.
Results
Patient characteristics
The median age of patients included in this trial was 57 years (range, 38−65 years) and the majority (94%) had an ECOG PS of 0−1. High-risk cytogenetics were present in all patients, with the most common being a t(4;14) translocation. In total, 68% of patients had two cytogenetic abnormalities (Table 1).
Table 1. Patient baseline characteristics and treatment disposition*
|
ASCT, autologous stem cell transplantation; ECOG PS, Eastern Cooperative Oncology Group performance status; R-ISS, revised International Staging System. |
|
|
Characteristic, % (unless otherwise stated) |
Total (N = 50) |
|---|---|
|
ISS score |
|
|
Stage 1 |
42 |
|
Stage 2 |
34 |
|
Stage 3 |
24 |
|
R-ISS score |
|
|
Stage 2 |
76 |
|
Stage 3 |
24 |
|
High-risk cytogenetics |
100 |
|
17p depletion |
40 |
|
t(4;14) |
52 |
|
t(14;16) |
20 |
|
1q gain |
50 |
|
Two high-risk cytogenetic abnormalities |
68 |
|
Treatment disposition |
|
|
Ongoing |
72 |
|
Consolidation |
22 |
|
ASCT #2, n |
1 |
|
Maintenance |
48 |
|
Discontinued |
28 |
|
Progressive disease, n |
2 |
|
Adverse event, n |
4 |
|
Withdrawal, n |
2 |
|
Stem cell collection failure, n |
6 |
Safety
Treatment-emergent adverse events (TEAEs) are shown in Figure 2. No new safety signals were observed in this trial for dara-KRd + tandem transplant. The most common hematologic TEAE was neutropenia, with infection and gastrointestinal disorders being the most frequently observed non‑hematologic TEAEs.
There were two cases of patients with AEs leading to discontinuation (one case of COVID-19 infection and one case of tumor lysis syndrome). Grade III/IV infections occurred in three patients:
- COVID-19 (n = 1)
- Cytomegalovirus infection (n = 1)
- Pseudomonas aeruginosa bacteriemia (n = 1)
Figure 2. Treatment-emergent adverse events*
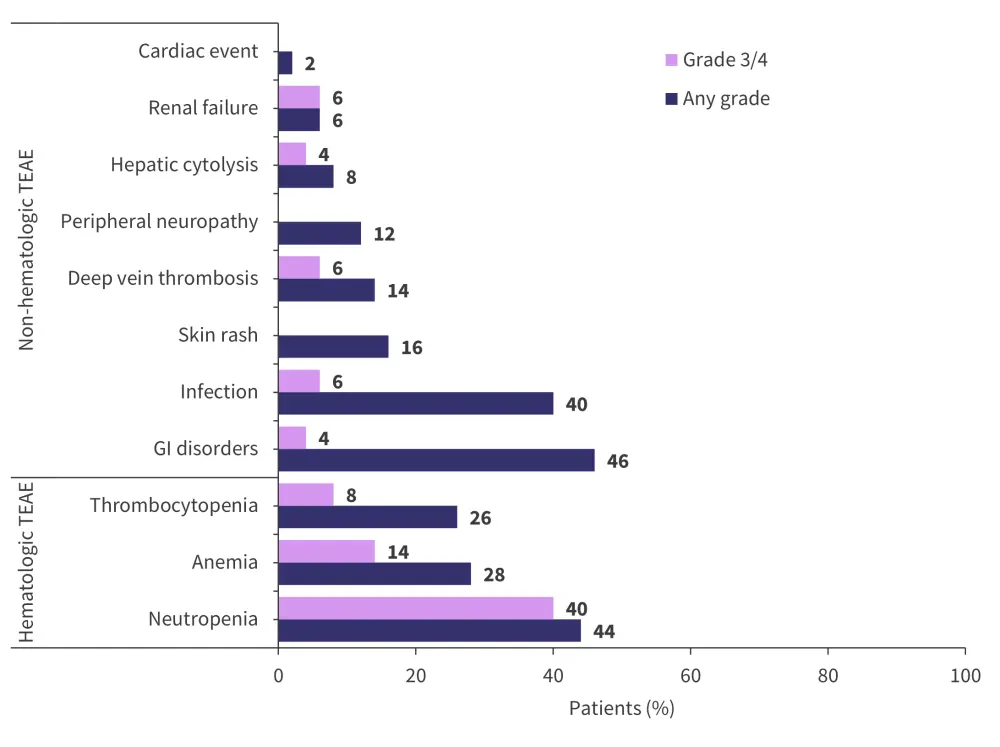
GI, gastrointestinal; TEAE, treatment-emergent adverse events.
*Adapted from Touzeau.1
Stem cell collection
During the first round of stem cell collection, failure occurred in six patients. In the patients in which collection was successful, the median CD34+ cell count was 6.1 × 106/kg (range, 0−16 × 106/kg).
As a result of this failure, the protocol was amended to collect stem cells after Cycle 3. Following this change, stem cell collection was successful in 21 patients and the median CD34+ cell collection achieved was 8.3 × 106/kg (range, 4.7−26 × 106/kg).
Response
Measurable residual disease (MRD) was measured by next-generation sequencing with a threshold of 10−5. MRD negativity rate was 62%. The response to treatment is shown in Figure 3. Complete response/stringent complete response rate was 31% and the very good partial response or better rate was 91%.
Figure 3. Response rate*
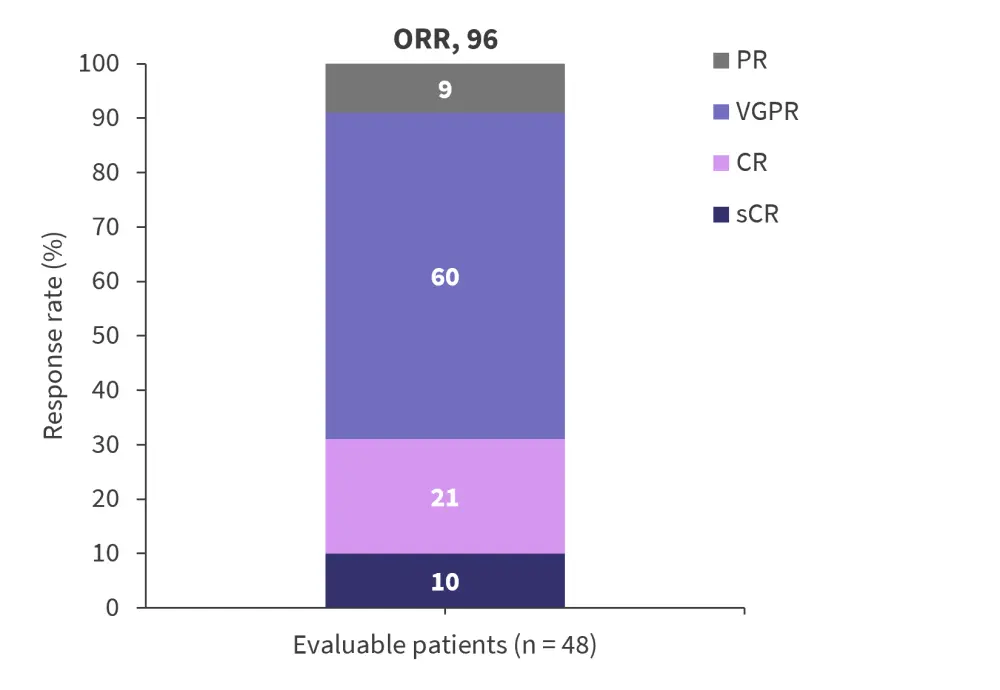
CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent CR; VGPR, very good partial response.
*Adapted from Touzeau.1
Median follow-up was 19.4 months and the survival outcomes for patients with NDMM are shown in Table 2. At all timepoints, median PFS and OS were >90%.
Table 2. Survival outcomes at 12 months and 18 months*
|
OS, overall survival; PFS, progression-free survival. |
|
|
Outcome, % (range) |
N = 50 |
|---|---|
|
PFS |
|
|
12 months |
96 (90−100) |
|
18 months |
92 (84−100) |
|
OS |
|
|
12 months |
96 (90−100) |
|
18 months |
96 (90−100) |
Conclusion
The results of the IFM-2018-04 trial in high-risk transplant-eligible patients with NDMM show that the quadruplet of dara-KRd + tandem transplant resulted in PFS and OS rates >90% at 18 months. An MRD-negativity rate of 62% was achieved with dara-KRd + tandem transplant, which was the same as the MRD-negative rate of 62% achieved in the FORTE trial with KRd and single transplant. These results are consistent with those achieved in the GMMG concept trial of isatuximab + KRd in high‑risk patients. To confirm both the efficacy and feasibility of this treatment combination and sequencing, a longer follow-up is required.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




