All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
ASH 2021 update from the GRIFFIN trial of dara-RVd in newly diagnosed patients with MM
Your opinion matters
What is your main concern regarding the current approaches evaluating anti-CD38 maintenance therapy?
During the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, Jacob Laubach1 presented an update of the phase II GRIFFIN trial (NCT02874742), investigating daratumumab (dara), lenalidomide, bortezomib, and dexamethasone (RVd) versus RVd alone in transplant-eligible patients with newly diagnosed multiple myeloma (NDMM). The Multiple Myeloma Hub previously reported on the GRIFFIN trial here and the study design is detailed here.
Results
Treatment discontinuation
This update provides data after patients received 2 years of maintenance therapy with dara + lenalidomide (dara-RVd arm), lenalidomide alone (RVd arm), or following treatment discontinuation. Around 20% of patients in the dara-RVd or RVd arm ceased treatment during maintenance therapy.
Treatment discontinuation during maintenance occurred due to:
- adverse events, which were balanced in both arms (8% vs 7% in dara-RVd vs RVd, respectively); or
- progressive disease, which was greater in the RVd arm (3% vs 7% in dara-RVd vs RVd, respectively).
Efficacy
The median follow-up was 38.6 months and responses were seen to deepen over time in both arms (Figure 1). There was a significant increase in the percentage of patients achieving a stringent complete response (CR; p = 0.0096) and ≥CR (p = 0.0013) in the dara-RVd arm compared with the RVd arm after 2 years of maintenance.
Figure 1. Responses over time in the dara-RVd and RVd arms*
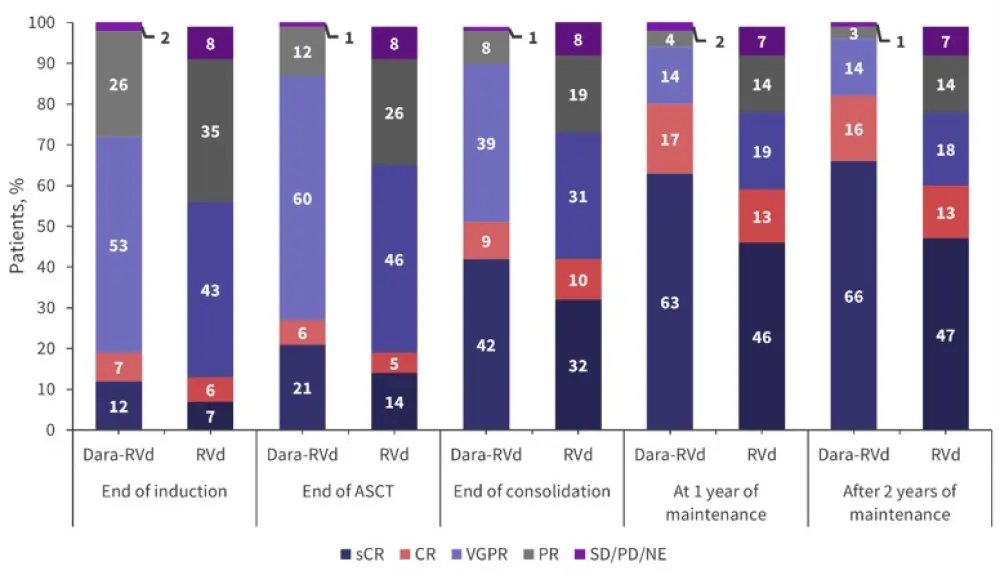
ASCT, autologous stem cell transplant; CR, complete response; dara-RVd, daratumumab, lenalidomide, bortezomib, and dexamethasone; NE, not evaluable; PD, disease progression; RVd, lenalidomide, bortezomib, and dexamethasone; sCR, stringent CR; SD, stable disease; VGPR, very good partial response.
*Adapted from Laubach et al.1
Measurable residual disease (MRD) negativity measured at a threshold of 10−5 was significantly higher in the dara-RVd arm (64%) compared with the RVd arm (30%; p < 0.0001) in the intention-to-treat population after 2 years of maintenance therapy. MRD negativity rates also improved over time, with 29% of the dara-RVd patients and 12% of the RVd patients who were MRD positive at the end of consolidation becoming negative by the end of 2 years of maintenance with dara-R or R (Figure 2). Improvement in MRD negativity rates was also reported between 1 year and 2 years of maintenance therapy.
Figure 2. MRD negativity (10−5) from the end of induction until 2 years of maintenance*
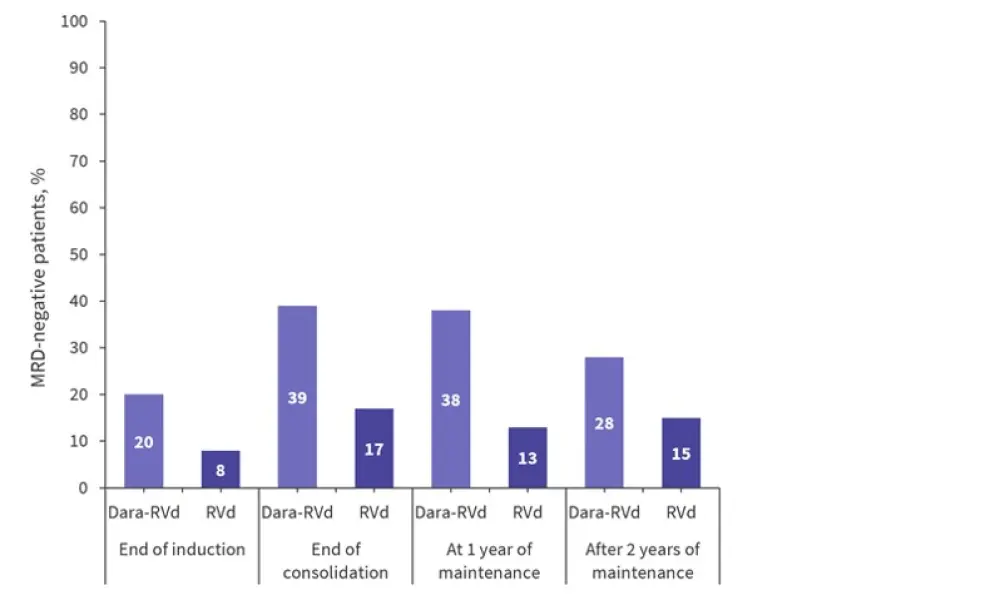
Dara-RVd, daratumumab, lenalidomide, bortezomib, and dexamethasone; MRD, measurable residual disease; RVd, lenalidomide, bortezomib, and dexamethasone.
*Adapted from Laubach et al.1
The percentage of patients with sustained MRD negativity at 10−5 after ≥6 and ≥12 months was significantly increased in the dara-RVd arm compared with the RVd arm (Figure 3). To date, no patient who sustained MRD negativity beyond 12 months has progressed.
Figure 3. MRD negativity (10−5) lasting ≥6 and ≥12 months in the dara-RVd and RVd arms*
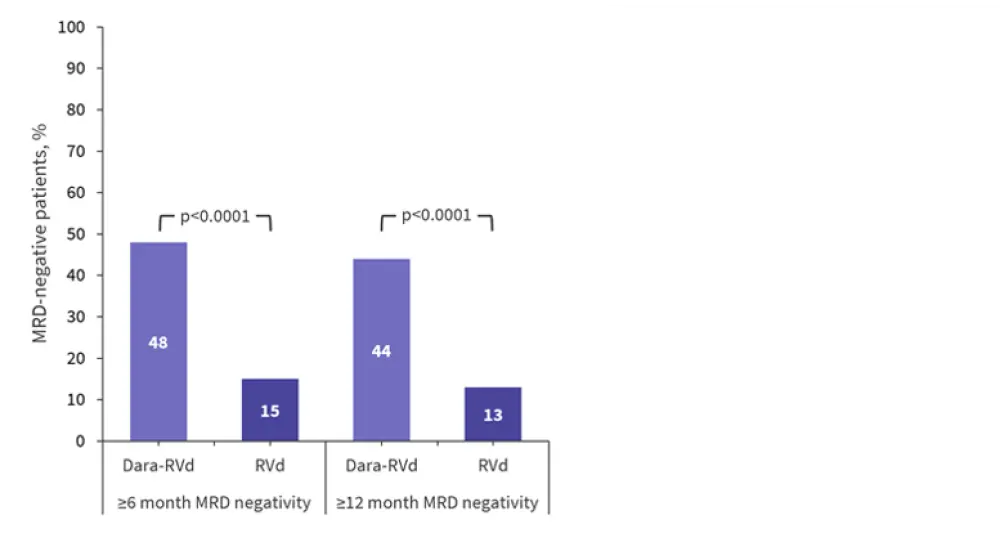
Dara-RVd, daratumumab, lenalidomide, bortezomib, and dexamethasone; MRD, measurable residual disease; RVd, lenalidomide, bortezomib, and dexamethasone.
*Adapted from Laubach et al.1
While the study was not powered to assess progression-free survival (PFS), there was a trend towards improved PFS with dara-RVd, showing a potential benefit of prolonged therapy with dara-R (3-year PFS rate, 88.9% in dara-RVd vs 81.2% in RVd; hazard ratio, 0.46; 95% confidence interval, 0.21–1.01). With a median follow-up of 38.6 months, median PFS and overall survival were not reached in either group. However, little difference has been seen between the two arms for overall survival so far.
Safety
Elevated rates of neutropenia (any grade, 64% vs 40%) and upper respiratory tract infection (any grade, 68% vs 50%) were reported in the dara-RVd arm vs RVd arm, respectively. No new safety concerns were seen in this extended follow-up. During maintenance, any-grade infection rates were similar between the patients treated with dara-RVd (36%) and RVd (32%) with ≥1 infection, with the most common being upper respiratory tract infection and pneumonia.
Conclusion
Dara-RVd used as an induction regimen produced a higher level of CR than RVd in patients with NDMM. MRD negativity rates were also seen to be greater in the dara-RVd arm than the RVd arm and these deepened over time. There was a positive trend towards increased PFS in the dara-RVd arm compared with the RVd arm, although the study was not powered to assess PFS. No new safety signals were observed following 2 years of maintenance with dara-R or R, and due to the continuous response improvement observed, patients were encouraged to stay on R maintenance therapy beyond the completion of the study treatment.
The use of dara-RVd as an induction agent in transplant-eligible patients with NDMM, followed by dara-R maintenance, is supported by the results of this trial. The ongoing phase III Perseus trial (NCT03710603) will further investigate dara-RVd with dara-R maintenance versus RVd with R maintenance in a larger cohort of patients with NDMM.
For more information on the potential role of dara as maintenance therapy, listen to our podcast with Jacob Laubach and Peter Voorhees regarding the latest updates from the GRIFFIN and CASSIOPEIA trials.
Daratumumab as maintenance therapy for transplant-eligible patients with NDMM: What have we learned from GRIFFIN and CASSIOPEIA trials?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

