All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Daratumumab plus VRd vs VRd in newly diagnosed MM: GRIFFIN trial 2020 analysis
Featured:
Update: Find the latest report on the GRIFFIN trial, here.
The standard induction treatment for patients with newly diagnosed multiple myeloma (NDMM) eligible for autologous stem cell transplant (ASCT) involves a combination of an immunomodulatory drug (lenalidomide or thalidomide), a proteasome inhibitor (bortezomib), and dexamethasone. Daratumumab, a human immunoglobulin G kappa (IgGκ) antibody that targets CD38-expressing cells, has recently been approved in combination with bortezomib/thalidomide/dexamethasone (VTd) for this patient population.
In efforts to increase the depth of response, including the rates of stringent complete response (sCR) and measurable residual disease (MRD) negativity, daratumumab is also being evaluated combined with bortezomib/lenalidomide/dexamethasone (D-VRd) in the GRIFFIN trial (NCT02874742).1,2
The study design and earlier results from this trial have previously been reported on the Multiple Myeloma Hub. Here, we report the outcome of the final analysis of the GRIFFIN safety run-in cohort and an update following 12 months of maintenance therapy, as published in Blood Advances and presented during the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.2,3
Patient disposition
A total of 16 patients were enrolled in the safety run-in cohort.2 All patients completed induction therapy, stem cell mobilization, ASCT, and consolidation and initiated maintenance therapy.
In the main study, 207 patients were randomized to the D-VRd (n = 104) and VRd (n = 103) arms.3 Of note, 16% and 14% of patients in the two respective groups had high-risk cytogenetics. At data cut-off, 87% (D-VRd) and 68% (VRd) of patients entered the maintenance phase, and 12% (D-VRd) and 17% (VRd) discontinued treatment, mainly due to adverse events and progressive disease.
Results
Responses recorded in the safety run-in cohort are shown in Table 1.2 Briefly, a CR was obtained in 12.5% of patients by the end of the induction period. By the end of the D-VRd consolidation period, a 56.3% sCR rate was reported. After a median of >3 years of follow-up, sCR rate was 93.8%. The median time to ≥CR was 7.36 months (range, 2.8–18.5), and the median duration was not estimable. MRD negativity at 10-5 and 10-6 by next-generation sequencing attained at the end of induction, consolidation, and last follow-up in the safety run-in cohort is also summarized in Table 1.
Table 1. Safety run-in cohort responses and MRD negativity rates for D-VRd treated patients*
| CR, complete response; D-VRd, daratumumab + VRd; MRD, measurable residual disease; sCR, stringent CR; PR, partial response; VGPR, very good partial response; VRd, bortezomib + lenalidomide + dexamethasone. *Data from Voorhees et al.2 †50% of patients achieved sustained MRD negativity at 10-5 for ≥12 months. |
|||
|
|
End of induction period, % |
End of consolidation period, % |
By last follow-up, % |
|---|---|---|---|
|
CR + sCR |
12.5 |
68.8 |
93.8 |
|
VGPR |
56.3 |
31.3 |
6.3 |
|
PR |
31.3 |
0 |
0 |
|
MRD 10-5 |
18.8 |
50 |
81.3† |
|
MRD 10-6 |
0 |
0 |
31.3 |
In the randomized part of the trial,3 responses also deepened over time. At 12 months of maintenance therapy cut-off, the median follow-up was 27.4 months. Data collected at that timepoint are presented in Figure 1. Results for end of induction, ASCT, and consolidation are based on a median follow-up of 13.5 months (primary analysis). A 30.3% increase in ≥CR rate was observed with D-R from the end of consolidation to ≥12-month maintenance, compared to an 18.5% increase in the control group (R maintenance only) during the same timeframe.
Figure 1. Patient responses to D-VRd vs VRd collected from the end of induction to the 12-month maintenance cut-off (median follow-up, 27.4 months)*
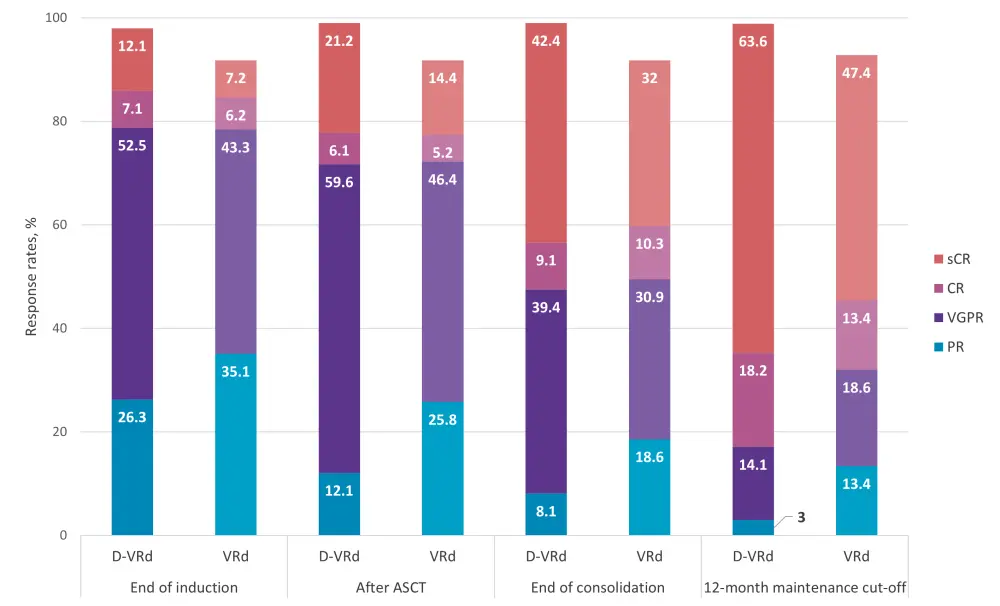
ASCT, autologous stem cell transplant; CR, complete response; D-VRd, daratumumab + VRd; SD, stable disease; PD, progressive disease; PR, partial response; VRd, bortezomib + lenalidomide + dexamethasone; VGPR, very good partial response.
*Adapted from Kaufman et al.3
MRD negativity was analyzed at the 12-month maintenance therapy cut-off in the intent-to-treat (ITT) population.3 MRD negativity was compared using various perspective analyses. Sustained MRD negativity (10-5) for ≥6 and ≥12 months in the ITT population treated with D-VRd was 37.5% and 28.8%, respectively. On the other hand, the VRd-treated cohort had 7.8% and 2.9% sustained MRD negativity rates at the same timepoints. Among patients with MRD negative status, sustained MRD negativity rate lasting more than 12 months was 46.2% (D-VRd) and 10.7% (VRd).
Subgroup analysis of MRD negativity revealed that the quadruplet had better outcomes than the triplet, and all subsets benefitted from the addition of daratumumab. Improved MRD negativity rates were also observed for patients with high-risk cytogenetics: 68.3% vs 28.6% in the D-VRd vs VRd groups.
For progression-free survival (PFS) and overall survival (OS) rates, no statistically significant differences were observed between the D-VRd and VRd arms (Table 2).
Table 2: PFS and OS outcomes for the safety run-in cohort and the randomized trial after ≥12 months of maintenance therapy
|
D-VRd, daratumumab + VRd; OS, overall survival; PFS, progression-free survival; VRd, bortezomib + lenalidomide + dexamethasone. |
||||
|
Outcome |
Timepoint, months |
Safety run-in cohort2 |
12–24 months maintenance therapy3 |
|
|---|---|---|---|---|
|
D-VRd |
D-VRd |
VRd |
||
|
PFS (%) |
12 |
— |
96.9 |
94 |
|
24 |
93.8 |
94.5 |
90.8 |
|
|
36 |
78.1 |
— |
— |
|
|
OS (%) |
12 |
— |
99 |
97.9 |
|
24 |
93.8 |
94.7 |
93.3 |
|
|
36 |
93.8 |
— |
— |
|
Safety
The most common treatment-emergent adverse events (TEAEs) observed in the safety run-in cohort were as follows2:
- Neutropenia (43.8%)
- Pneumonia (31.3%)
- Lymphopenia (31.3%)
- Thrombocytopenia (25.0%)
- Hypertension (18.8%)
- One patient had TEAEs leading to discontinuation of lenalidomide and bortezomib
In the randomized and larger part of the trial, no new safety issues have been identified to date.3 Neutropenia and upper respiratory tract infections were common TEAEs in both arms, and were more frequently observed in the D-VRd cohort. However, this did not lead to an increased rate of discontinuation. During maintenance therapy, a total of 53% of patients experienced an upper respiratory tract infection of any grade in the D-VRd group compared to 41% of patients in the VRd group. The occurrence of pneumonia during maintenance was found to be similar for both groups: 13% (D-VRd) and 15% (VRd).
Conclusion
Treatment of patients with D-VRd induction followed by ASCT, D-VRd consolidation, and D-R maintenance treatment resulted in better responses and improved depth of responses compared to VRd followed by R maintenance. The sCR and MRD negativity rates significantly improved with maintenance therapy. PFS and OS rates are promising, but it is still too soon to see differences.
These results obtained after 12 months of maintenance therapy reveal the potential of D-VRd and D-R maintenance as a new treatment for patients with NDMM who are eligible for transplant. The ongoing phase III trial, PERSEUS (NCT03710603), further evaluates the safety and efficacy of D-VRd in this patient population.
The phase II GRIFFIN trial was one of the main talking points in a recent podcast by Kaufman and Gay. Listen below to the discussion about identifying the best partner for lenalidomide in maintenance therapy after ASCT in MM.
ASH 2020 discussion: Looking for the best partner for lenalidomide maintenance after auto-SCT
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Jonathan L. Kaufman
Jonathan L. Kaufman Francesca Gay
Francesca Gay