All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Teclistamab: real-world safety and efficacy
Do you know... The real-world analysis focused on patients with high-risk disease characteristics. What percentage of patients treated with teclistamab experienced a complete response or better?
Teclistamab is a T-cell directed bispecific antibody that binds to CD3 and B-cell maturation antigens on plasma cells. Results from the phase II part of the MajesTEC-1 trial (NCT04557098) resulted in the approval of teclistamab by the U.S. Food and Drug Administration (FDA) in October 2022 for the treatment of relapsed/refractory multiple myeloma. While results from the trial were positive, the stringent inclusionary criteria limited the representation of real-world patients, who commonly present organ dysfunction, significant comorbidities, and high-risk disease characteristics.
During the 65th American Society of Hematology Annual Meeting and Exposition, Dima presented results from a retrospective analysis to evaluate the real-world safety and efficacy of teclistamab, with a focus on patients who would have been ineligible for the MajesTEC-1 trial. We summarize the key findings below.
Check out our top abstracts presented at the 65th ASH Annual Meeting and Exposition here. For more information on the incidence and management of infections in patients enrolled in the MajesTEC-1 trial, read our previous Multiple Myeloma Hub article.
Listen to this article as a podcast here:
Teclistamab: real-world safety and efficacy
Study design1
- The full study design of the MajesTEC-1 trial was reported in this Multiple Myeloma Hub article.
- This study was a retrospective multicenter analysis of the expanded-access program for teclistamab, which took place between August 2022 and August 2023.
- All patients had received at least one full dose of teclistamab as of July 15, 2023
- The data cutoff was August 15, 2023.
Results1
- The baseline patient characteristics and reasons for MajesTEC-1 ineligibility are shown in Table 1.
Table 1. Baseline patient characteristics and reasons for MajesTEC-1 ineligibility*
|
ASCT, autologous stem cell transplant; BCMA, B-cell maturation antigen; CNS, central nervous system; ECOG, |
|
|
Characteristic, % (unless otherwise specified) |
N = 106 |
|---|---|
|
Median age, years |
66.5 |
|
Median time since diagnosis, years |
5.5 |
|
Median number of prior lines of therapy, n |
6 |
|
>4 prior lines of therapy |
80 |
|
R-ISS stage III |
31 |
|
ECOG performance status ≥2 |
33 |
|
EMD |
42 |
|
High-risk cytogenetics |
59 |
|
Prior BCMA therapy |
53 |
|
Reasons for MajesTEC-1 ineligibility, % (unless otherwise specified) |
n = 88 |
|
Prior anti-BCMA therapy |
53 |
|
Poor performance status (ECOG ≥2) |
33 |
|
Any baseline cytopenia |
31 |
|
Renal dysfunction |
13 |
|
Liver dysfunction |
5 |
|
Cardiac dysfunction |
4 |
|
Plasma cell leukemia |
2 |
|
Amyloidosis |
1 |
|
CNS involvement |
4 |
|
ASCT ≤12 weeks |
1 |
- A total of 56 patients had received prior B-cell maturation antigen therapy
Efficacy1
- The real-world efficacy results of teclistamab are shown in Figure 1.
Figure 1. Real-world efficacy results*
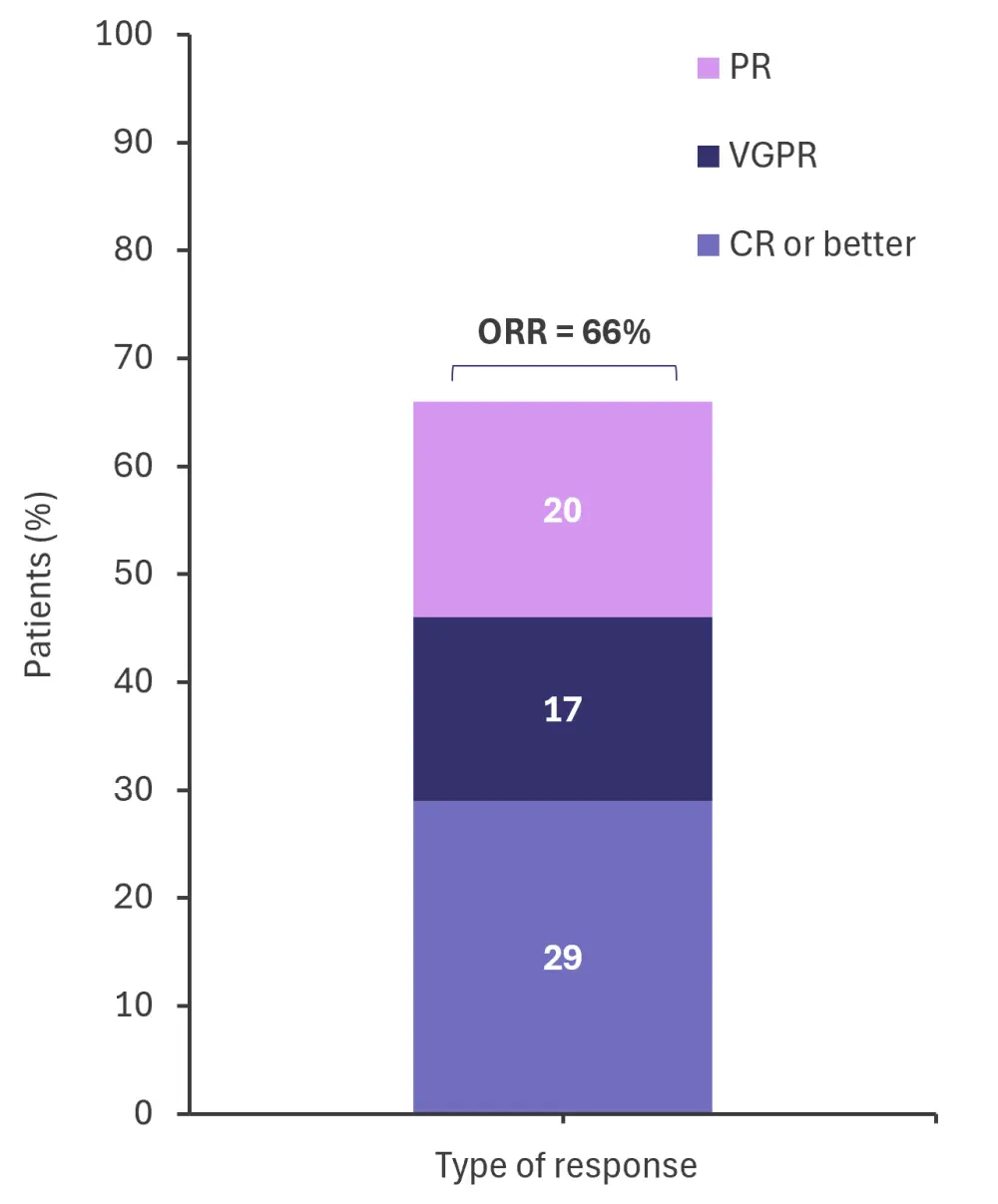
CR, complete response; ORR, overall response rate; PR, partial response; VGPR, very good PR.
*Adapted from Dima, et al.1
- The overall response rates (ORR) according to the type of previous therapy are shown in Table 2.
Table 2. ORR according to previous therapy*
|
ADC, antibody-drug conjugate; CAR T-cell, chimeric antigen receptor T-cell; ORR, overall response rate. |
||
|
Type of previous therapy |
ORR |
Number of patients |
|---|---|---|
|
ADC |
50 |
10 |
|
CAR T cell |
57 |
33 |
|
ADC + CAR T cell |
80 |
10 |
|
ADC + other |
33 |
3 |
- Median follow-up time was 3.8 months.
- Median progression-free survival (PFS) was 5.4 months.
- Median overall survival (OS) was not reached:
- 6-month OS rate 70%; and
- 10-month OS rate 67%.
- Multivariate analysis revealed several factors were independently associated with inferior ORR; these were:
- European Cooperative Oncology Group (ECOG) performance score ≥2;
- extramedullary disease; and
- >4 prior lines of therapy.
- Factors independently associated with inferior PFS were:
- ECOG performance score ≥2;
- extramedullary disease; and
- age ≤70 years.
Safety1
- Cytokine release syndrome of any grade was experienced by 64% of patients, with a median duration of 1 day.
- Immune effector cell-associated neurotoxicity of any grade was experienced by 14% of patients, with a median duration of 2 days.
- The percentage of patients who experienced hematological adverse events of any grade at Days 30 and 90 are shown in Figure 2.
Figure 2. Any-grade hematological adverse events at Day 30 and Day 90*
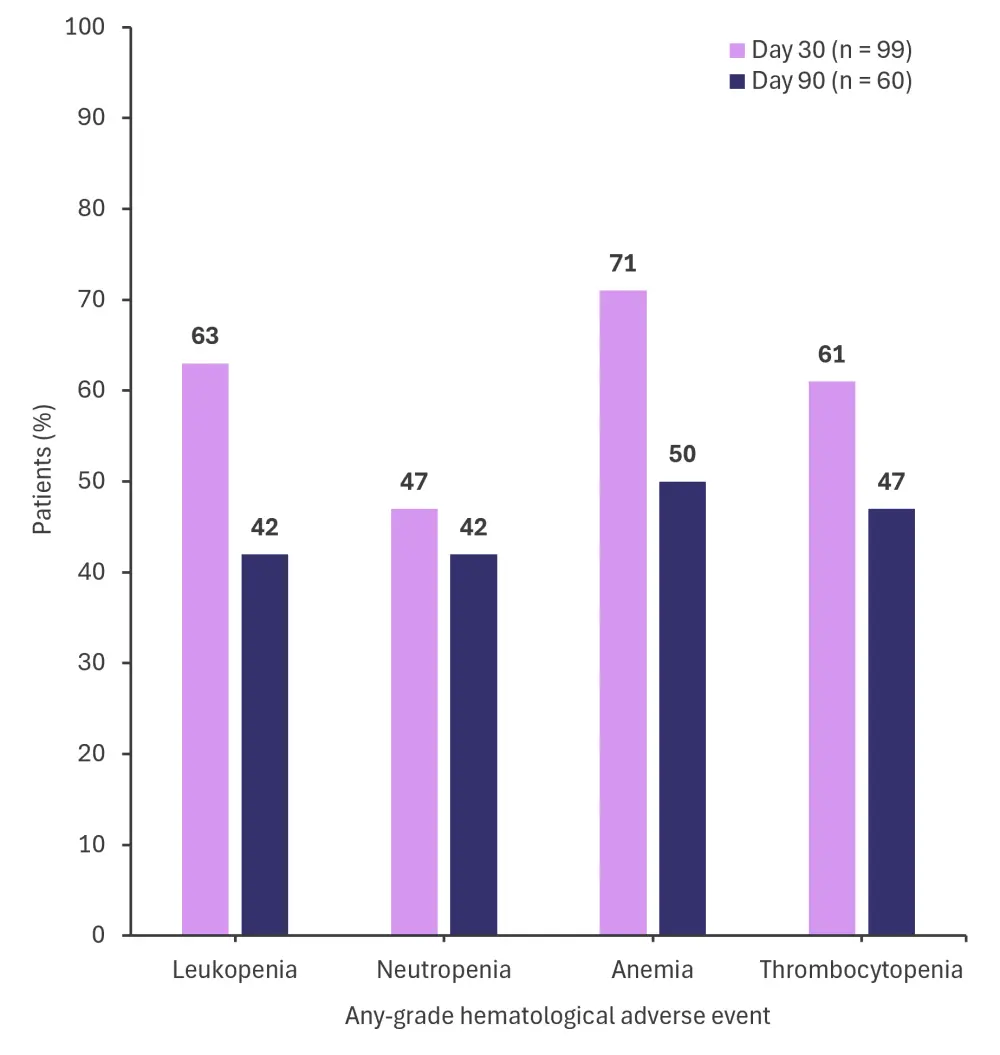
*Adapted from Dina, et al.1
- A total of 39 infections were reported, of which 46% were serious.
- In total, 29 patients died during the study.
- The main cause of death was disease progression (86%).
Conclusion1
Teclistamab demonstrated efficacy and favorable tolerability in the real-world setting, especially in patients with heavily pretreated relapsed/refractory disease. The ORRs were similar to those reported in the MajesTEC-1 trial. However, lower rates of complete response or better were seen in this setting. Extramedullary disease and ECOG performance score independently predicted worse OS and PFS. Incidences of severe cytokine release syndrome and immune cell-associated neurotoxicity were minimal. Future research should explore the risk-benefit balance of shorter treatment durations and potential cessation in patients achieving deep remission to support immune recovery.
This educational resource is independently supported by Johnson & Johnson . All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




