All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
MajesTEC-1: Incidence and management of infections in patients with RRMM treated with teclistamab
Do you know... In a detailed analysis of the MajesTEC-1 study by Nooka et al., what percentage of patients with relapsed/refractory multiple myeloma treated with teclistamab experienced infections?
Patients with relapsed/refractory multiple myeloma (RRMM) are more vulnerable to infections due to immune dysregulation caused by multiple drugs involved in treatment. In October 2022, teclistamab was approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with RRMM based on the results of the phase I/II MajesTEC-1 study. The primary safety analysis from the MajesTEC-1 study (NCT03145181 and NCT04557098) showed that infections occurred in 76.4% of patients, with 44.8% of Grade 3–4.
Recently Nooka et al.1 published a detailed analysis of MajesTEC-1 in Cancer, providing recommendations for the prevention and management of potential infections during treatment with teclistamab. The Multiple Myeloma Hub is pleased to summarize the key findings here.
Study design
The study and patient characteristics have previously been reported by the Multiple Myeloma Hub. For the current analysis, overall infections were analyzed as well as selected clinically relevant infections. Table 1 shows the history of infections in included patients.
Table 1. Baseline history of infections*
|
*Data from Nooka, et al.1 |
||
|
Infection history, % |
Total |
Ongoing at screening |
|---|---|---|
|
Infections† |
33.3 |
7.9 |
|
Pneumonia |
7.9 |
0 |
|
Herpes zoster |
4.2 |
0 |
|
COVID-19 |
3.6 |
0 |
|
Sinusitis |
2.4 |
1.2 |
|
Upper respiratory tract infection |
2.4 |
0 |
|
Diverticulitis |
1.8 |
0.6 |
|
Urinary tract infection |
1.8 |
0.6 |
|
Hepatitis A |
1.2 |
0 |
|
Hepatitis B |
1.2 |
0 |
|
Nasopharyngitis |
1.2 |
0.6 |
|
Onychomycosis |
1.2 |
0.6 |
Results
At a median follow-up of 22.8 months (range, 0.3–33.6 months), a total of 165 patients were included. Patients received a median number of five (range, 2–14) prior lines of therapy.
Incidence of infections
Overall, infections were reported in 80% of patients, including opportunistic infections in 9.1% of patients. Figure 1 shows the incidence of overall infections, including Grade 3–4 infections.
Figure 1. Incidence of infections*
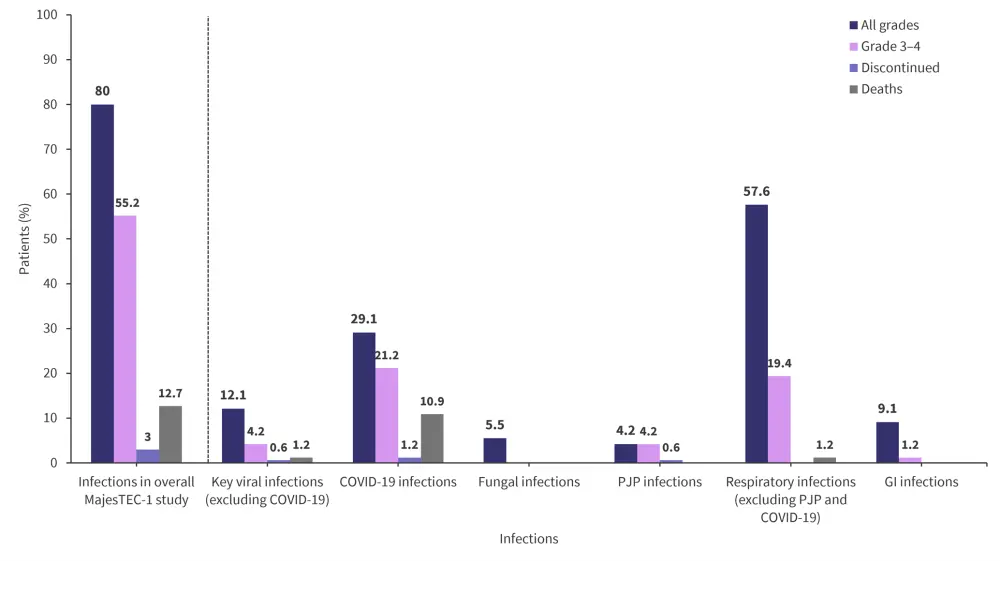
GI, gastrointestinal infection; PJP, Pneumocystis jirovecii pneumonia.
*Data from Nooka, et al.1
Timing of infections
- The median time to first onset of any grade and Grade 3–4 infections infection was 1.7 months (range, 0–24.7 months) and 4.2 months (range, 0–34.6 months), respectively
- Most Grade 3–4 infections occurred within the first 2 months of receiving teclistamab administration
- A total of 21 deaths due to infections were reported, of which 18 were due to COVID-19
- Incidence of Grade ≥3 infections reduced over time, particularly in patients who switched to bi-weekly teclistamab dosing
- More than half of viral infections of any grade occurred within 6.6 months of teclistamab administration
- Following treatment with teclistamab,
- Grade 2 adenoviral reactivation occurred in one patient after 2.4 months;
- Grade 3 cytomegalovirus reactivation occurred in one patient after 4.2 months;
- Grade 3 hepatitis B virus reactivation occurred in one patient after 3.5 months; and
- Grade 3 cytomegalovirus viremia occurred in two patients after 1.2 months and 10.7 months, respectively.
- Following treatment with teclistamab,
- COVID-19 infections were managed by supportive care and/or interruption of teclistamab treatment
- Patients discontinued treatment at 20.7 months and 16.4 months due to Grade 3 and Grade 4 COVID-19 infections, respectively
- Deaths occurred earlier in unvaccinated patients compared with vaccinated patients (0.7–5.9 months vs 4.3–25.9 months)
- Grade 2 Candida infections occurred in four patients after 1 month of receiving teclistamab, except in one patient after 14.8 months
- Grade 2 Aspergillus infection of Grade 2 occurred in one patient after 1 month
- Following treatment with teclistamab, seven patients developed pneumocystis jirovecii pneumonia infections after 2.5–7.8 months (duration, 8–60 days)
- Pneumonia was the most frequently occurring respiratory infection
- Three patients developed Grade 3 pseudomonal pneumonia after 2.5–17.7 months of receiving teclistamab which was resolved within 10–17 days
- Gastrointestinal infections occurred in 15 patients within the first 13 months of receiving teclistamab
- Most were Grade 1–2, except one patient who developed Grade 3 infectious enterocolitis after 9.6 months and one who developed Grade 3 diverticulitis after 2.6 months
Management and recommendations
Figure 2 outlines recommendations for the screening, management, and monitoring of patients receiving teclistamab treatment.
Figure 2. Recommendations for infection management*

CMV, cytomegalovirus; DNA, deoxyribonucleic acid; EBV, Epstein-Barr virus; HBc, hepatitis B core antibody; HBs, hepatitis B surface antibody; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; RNA, ribonucleic acid; VZV, varicella zoster virus.
*Adapted from Nooka, et al.1
Conclusion
Analysis of the MajesTEC-1 study demonstrated a slightly higher incidence of infections; however, the median time to onset of infection with teclistamab was similar to other B-cell maturation antigen bispecific antibodies. Based on these findings, close and continuous monitoring of patients treated with teclistamab is recommended. Patients are also encouraged to actively report any signs and symptoms of infection to facilitate prompt investigation and intervention. Of note, this study was limited by a small sample size and lack of statistical testing.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



