All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Results of a phase I/II study investigating belantamab mafodotin with lenalidomide and dexamethasone as frontline therapy
At the European Hematology Association (EHA)2022 Congress, updated safety and efficacy data from the ongoing phase I/II BelaRd study (EAE-2020; NCT04808037) were presented by Evangelos Terpos on behalf of the Hellenic Society of Hematology.1 The BelaRd study is assessing the safety and efficacy of belantamab mafodotin (belamaf) in combination with lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma who are ineligible for transplant, and it is the first study to evaluate belamaf as an upfront treatment.1
Previously, belamaf has shown anti-myeloma activity as a single agent in patients with relapsed/refractory multiple myeloma, and it was granted accelerated approval by the U.S. Food and Drug Administration (FDA) in August 20202 and approved by the European Medicines Agency (EMA) also in August 2020.3
Study design1
At the point of data cut-off, the BelaRd study had recruited 36 patients who were randomized equally into the three dosing cohorts below.
- Cohort 1 received 2.5 mg/kg belamaf
- Cohort 2 received 1.9 mg/kg belamaf
- Cohort 3 received 1.4 mg/kg belamaf
All cohorts received belamaf every 8 weeks, with 25 mg/day lenalidomide given orally on Days 1–21 of each 28-day cycle and 40 mg/day dexamethasone given orally or intravenously on Days 1, 8, 15, and 22 of each 28-day cycle. Key selection criteria for the study are shown in Table 1.
Table 1. Inclusion and exclusion criteria for the BelaRd study*
|
ASCT, autologous stem cell transplant; ECOG PS, Eastern Cooperative Oncology Group performance status; eGFR, estimated glomerular filtration rate. |
|
|
Inclusion criteria |
Exclusion criteria |
|---|---|
|
Ineligible for high-dose chemotherapy with ASCT |
Peripheral neuropathy or neuropathic pain of Grade 2 or higher† |
|
ECOG PS 0–2 |
Current corneal epithelial disease (except for mild punctate keratopathy) |
|
Adequate organ function |
|
|
eGFR ≥30 mL/min/1.73m2 |
|
The primary endpoint of the study is to evaluate the safety and tolerability of belamaf plus Rd, with the aim to establish a recommended phase II dose. Other endpoints include efficacy, pharmacokinetics, and corneal and ocular adverse events.
Results1
Patient characteristics of all three cohorts are shown in Table 2.
Table 2. Baseline patient characteristics*
|
ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, International staging system. |
|||
|
Characteristic, % (unless otherwise stated) |
Cohort 1 |
Cohort 2 |
Cohort 3 |
|---|---|---|---|
|
Median age (range), years |
75.0 |
74.5 |
69.0 |
|
Male |
66.7 |
41.7 |
50.0 |
|
ECOG PS |
|
|
|
|
0 |
33.3 |
25.0 |
66.7 |
|
1 or 2 |
66.7 |
75.0 |
33.3 |
|
ISS stage |
|
|
|
|
I |
33.3 |
25.0 |
33.3 |
|
II |
41.7 |
58.3 |
58.3 |
|
III |
25.0 |
16.7 |
8.3 |
|
Presence of high-risk cytogenetics† |
8.3 |
16.7 |
0.0 |
|
Ocular comorbidities |
|
|
|
|
Cataract, any grade |
83.3 |
83.3 |
91.7 |
|
Abnormal fundoscopic findings |
100.0 |
91.7 |
91.7 |
|
Abnormal intraocular pressure/glaucoma |
8.3 |
25.0 |
16.7 |
Safety
At the time of data cut-off (April 13, 2022), the following safety results were observed.
- The median duration of therapy was
- 3.9 months in cohort 1;
- 6.6 months in cohort 2; and
- 5.6 months in cohort 3.
- Two patients from cohort 1 and two patients from cohort 3 discontinued treatment.
- Two patients from cohort 1 and one patient from cohort 3 died, including one case caused by pneumonia and two cases due to COVID-19, not related to belamaf treatment.
- All but three patients experienced at least one treatment-emergent adverse event (TEAE) of any grade related to belamaf treatment, all of which were ocular AEs.
- The number of patients with ≥1 Grade 3/4 TEAE related to belamaf treatment was
- 6 in cohort 1 (4 TEAEs were ocular);
- 2 in cohort 2 (1 TEAE was ocular); and
- 3 in cohort 3 (all TEAEs were ocular).
- The most common non-ocular TEAEs (any grade) included fatigue, rash, diarrhea, neutropenia, leukopenia, and COVID-19 infection.
Efficacy
After a median follow-up of 4.2 months, the overall response rate was 91.7% in cohorts 1 and 2, and 100% in cohort 3. Response rates are shown in Figure 1.
Figure 1. Overall response rates depending on belamaf dose*
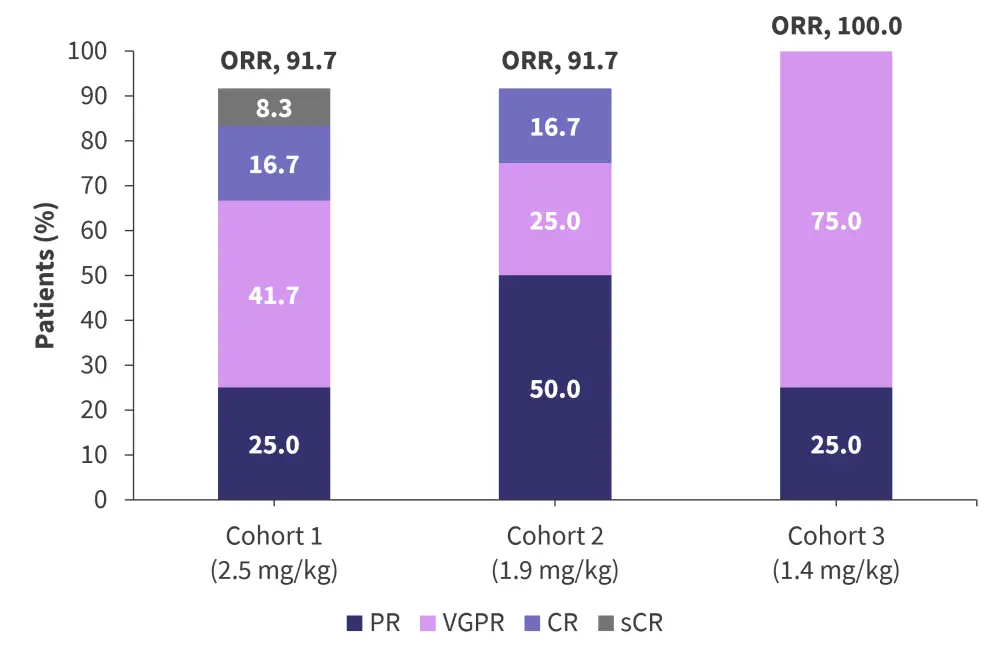
Belamaf, belantamab mafodotin; CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Terpos.
Conclusions and future perspectives
Given the low incidence of Grade 3/4 ocular TEAEs in cohort 2, the recommended phase II dose in this setting has been set at 1.9 mg/kg every 8 weeks. At this dose, the safety profile was considered manageable, with a complete response rate of 16.7% and overall response rate of 91.7%, suggesting belamaf plus Rd could be used in patients with newly diagnosed multiple myeloma not eligible for transplant.
The efficacy of belamaf seen in the relapsed setting has provided rationale to initiate several clinical trials in the first-line setting, as listed below.
- DREAMM 9 (NCT04091126) is phase I trial of belamaf in combination with bortezomib and Rd to determine the dosing schedule for a phase III trial.
- GEM-BELA-VRd (NCT04802356) is a phase II trial investigating belamaf as part of induction, consolidation, and maintenance therapy for patients with newly diagnosed multiple myeloma who are eligible for transplant.
- The NCT04876248 trial is studying belamaf as consolidation and maintenance therapy for patients with minimal residual disease after autologous transplant.
- EAE 120 (NCT05280275), another trial run by the Hellenic Society of Hematology, is investigating belamaf combined with daratumumab plus Rd.
These and additional ongoing studies should provide further insight into preventing the ocular toxicities associated with belamaf and enhancing its efficacy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



