All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Mezigdomide with bortezomib or carfilzomib for RRMM
Mezigdomide is an oral cereblon E3 ligase modulator (CELMoD) that induces apoptosis in multiple myeloma cells, creating a tumoricidal effect.1 The Multiple Myeloma Hub previously spoke to Paul Richardson about CELMoD; you can listen to the podcast here.
The first-in-human phase I dose escalation trial of mezigdomide, the CC-92480-MM-001 trial, showed promising preliminary results when mezigdomide was used in combination with dexamethasone, with an overall response rate of 55%.1 At the 19th International Myeloma Society Annual Meeting, Paul Richardson presented results from the CC-92480-MM-002 phase I/II dose escalation trial, where mezigdomide is evaluated in combination with dexamethasone and a proteasome inhibitor.1 We are pleased to provide a summary of this presentation here.
Study design
Phase I of the trial involved dose escalation, with patients receiving a 1 mg dose of mezigdomide in combination with two other drugs. Phase II of the trial involved dose expansion, using the results from phase I to determine the dosing regimen. In total, there were ten cohorts in this trial, evaluating mezigdomide with bortezomib, carfilzomib, daratumumab, elotuzumab, and isatuximab. The phase I results from Cohort A (mezigdomide with bortezomib and dexamethasone; Mezi-Vd) and Cohort C (mezigdomide with carfilzomib and dexamethasone; Mezi-Kd) and phase II results from Cohort D (Mezi-Vd) are shown in this article.
Dosing schedules for Cohorts A, C and D are shown in Figure 1. The primary endpoints of the trial were to determine a dose and regimen (phase I) and to evaluate the safety and overall response rate of the regimen.
Figure 1. Dosing schedule in Cohorts A, C, and D*
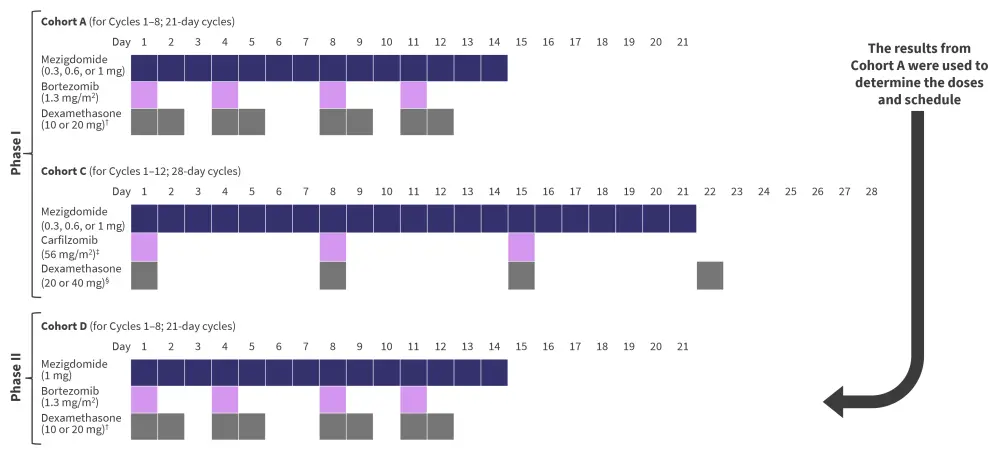
*Adapted from Richardson.1
†10 mg given if >75 years old.
‡On Cycle 1 Day 1, 20 mg/m2 was given.
§20 mg given if >75 years old.
Cycles could be continued past the number shown in Figure 1, with some with schedule adjustments. Adjustments were:
- In Cohort A and Cohort D, for Cycle 9 onwards, bortezomib was given on Day 1 and Day 8 only, and dexamethasone was given on Day 1, 2, 8 and 9 only.
- In Cohort C, for Cycle 13 onwards, carfilzomib was given on Day 1 and Day 15 only.
Results
Patient characteristics are shown in Table 1. A high proportion of patients were refractory to lenalidomide treatment. In general, Cohort D patients were less refractory and had received fewer lines of therapy compared to Cohort A and C. By protocol, patients in Cohort D had to be responsive to pomalidomide.
Table 1. Baseline patient characteristics*
|
ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, International Staging System; mAb, monoclonal antibody; MeziKd, mezigdomide, carfilzomib, and dexamethasone; MeziVd, mezigdomide, bortezomib, and dexamethasone. |
|||
|
Patient characteristic, % (unless otherwise stated) |
Cohort A |
Cohort C |
Cohort D |
|---|---|---|---|
|
Median age (range), years |
65.5 (46–86) |
68 (41–76) |
64 (43–83) |
|
Male |
42.9 |
34.6 |
71.1 |
|
ECOG PS |
|
|
|
|
0 |
39.3 |
38.5 |
39.5 |
|
1 or 2 |
60.7 |
61.5 |
60.5 |
|
ISS at study entry |
|
|
|
|
I |
71.4 |
76.9 |
68.4 |
|
II |
21.4 |
11.5 |
21.1 |
|
III |
7.1 |
11.5 |
10.5 |
|
Extramedullary disease |
17.9 |
3.8 |
7.9 |
|
High-risk cytogenetics† |
39.3‡ |
53.8§ |
34.2‖ |
|
Median prior therapies (range), n |
3 (2–4) |
2 (2–4) |
1 (1–3) |
|
Lenalidomide refractory |
85.7 |
80.8 |
65.8 |
|
Anti-CD38 mAb refractory |
50.0 |
73.1 |
36.8 |
|
Triple-class refractory¶ |
32.1 |
34.6 |
2.6 |
Safety
At the point of data cut-off (July 18, 2022), 53.6%, 42.3%, and 42.1% of patients had discontinued treatment in Cohort A, C, and D respectively. Discontinuation due to disease progression was 32.1% for Cohort A, 15.4% for Cohort C, and 26.3% for Cohort D. One patient died in Cohort C, which was due to sepsis and was not treatment related.
The most common treatment-emergent adverse events (any grade) were:
- infections (53.6%), neutropenia (50%), anemia (39.3%), and diarrhea (39.3%) in Cohort A;
- neutropenia (42.3%), infections (42.3%), and diarrhea (38.5%) in Cohort C; and
- neutropenia (71.1%), infections (65.8%), thrombocytopenia (47.4%), anemia (39.5%), and peripheral sensory neuropathy (39.5%) in Cohort D.
Discontinuation due to adverse events was 7.1% in Cohort A, 15.4% in Cohort C, and 13.2% in Cohort D. In total, 21.4%, 23.1%, and 36.8% of patients require a dose reduction of mezigdomide in Cohorts A, C, and D, respectively.
Efficacy
The median duration of response was:
- 14.5 months in Cohort A;
- 11.9 months in Cohort C; and
- not reached in Cohort D.
Partial response rates were similar across Cohorts A, C, and D. Very good partial response and complete response was higher in Cohort D compared with the other two cohorts. The overall response rates for Cohorts A, C, and D are shown in Figure 2.
Figure 2. Response rates across Cohorts A, C and D*
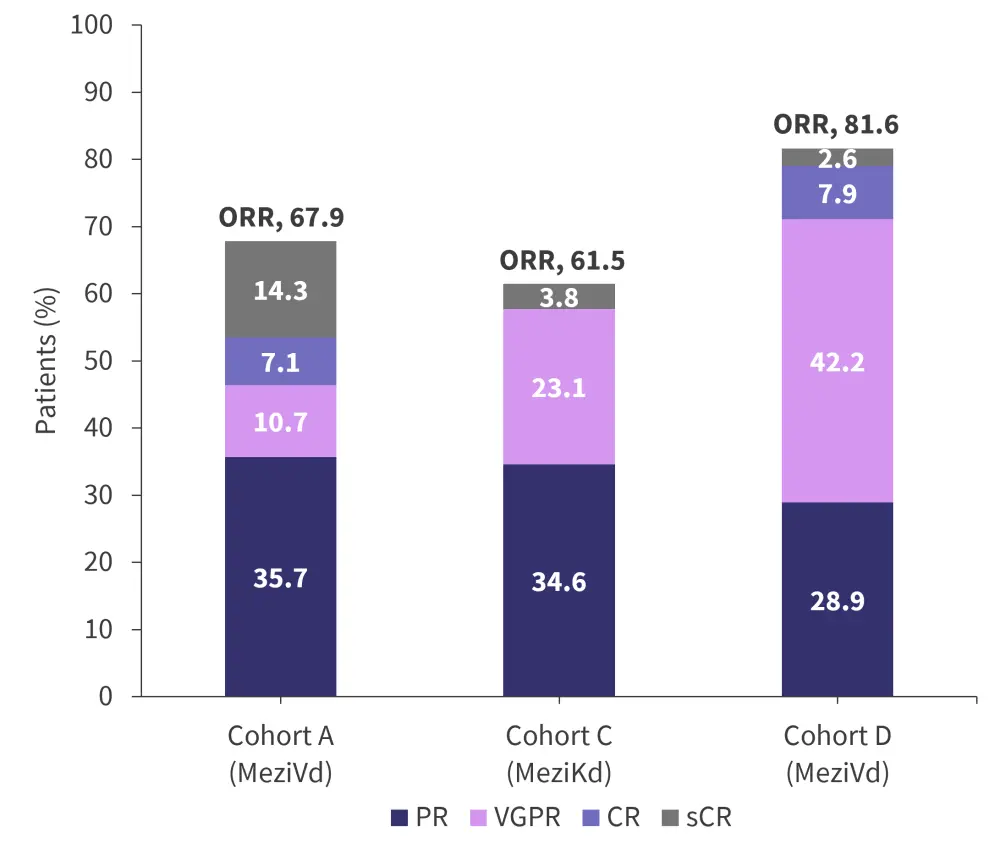
CR, complete response; MeziKd, mezigdomide, carfilzomib, and dexamethasone; MeziVd, mezigdomide, bortezomib, and dexamethasone; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Richardson.1
Conclusion
In Cohorts A, C, and D, mezigdomide was well tolerated, with low levels of discontinuation due to adverse events. The overall response rate of 81.6% in Cohort D, with over 50% of patients achieving very good partial response or better, suggests higher doses mezigdomide in combination with bortezomib and dexamethasone can be an effective treatment option for patients with relapsed/refractory multiple myeloma. However, 36.8% of patients in Cohort D required a dose reduction, which highlights the need for further trials to determine the optimum mezigdomide dose and schedule, while maintaining high efficacy. The combination used in Cohort C (mezigdomide with carfilzomib and dexamethasone) is also being continued to phase II, although results are not yet available.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



