All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Latest updates on cilta-cel from CARTITUDE-1 and CARTITUDE-4
Ciltacabtagene autoleucel (cilta-cel) is a B-cell maturation antigen (BCMA)-directed autologous chimeric antigen receptor (CAR) T-cell therapy.1 Cilta-cel is currently approved by the U.S. Food and Drug Administration (FDA) and the European Commission (EC) for the treatment of patients with relapsed/refractory multiple myeloma (MM) after four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. The Multiple Myeloma Hub has previously reported on these approvals, here and here, which were supported by data from CARTITUDE-1 (NCT03548207).
Recently, based on data from CARTITUDE-4 (NCT04181827), applications have been made to the U.S. FDA for a supplemental Biologics License Application and to the Europeans Medicine Agency (EMA) for the indication expansion of cilta-cel to include patients with relapsed and lenalidomide-refractory MM after one to three prior lines of therapy.2,3 Here, we are pleased to summarize the key data of both trials.
CARTITUDE-14
At the European Hematology Association (EHA) 2023 Hybrid Congress, Nikhil Munshi presented final 3-year follow up data from CARTITUDE-1. The study design and interim data have been previously reported on the Multiple Myeloma Hub.
Key endpoint data included an overall response rate of 97.9%, with 82.5% achieving stringent complete response. Median progression-free survival was 34.9 months and median overall survival (OS) was not reached; however, at 36 months, OS is estimated to be 62.9%. Patients from the CARTITUDE-1 trial will continue to be assessed for safety and survival outcomes in the 15-year long-term CARTINUE study (NCT05201781).
CARTITUDE-4
Study design
CARTITUDE-4 is a phase III randomized trial assessing the safety and efficacy of cilta-cel versus standard-of-care (SOC) pomalidomide, bortezomib, and dexamethasone or daratumumab, pomalidomide, and dexamethasone in adult patients with lenalidomide-refractory MM after one to three prior lines of therapy.1
The study design and primary and secondary endpoints are outlined in Figure 1.
Figure 1. Study design*

BCMA, B-cell maturation antigen; CAR-T, chimeric antigen receptor therapy; cilta-cel, ciltacabtagene autoleucel; DPd, daratumumab, pomalidomide, and dexamethasone; ECOG PS, Eastern Cooperative Oncology Group Performance Status; IMiD, immunomodulatory agent; len, lenalidomide; LOT, line of therapy; MRD, minimum residual disease; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PI, proteasome inhibitor; PRO, patient reported outcomes; PVd, pomalidomide, bortezomib, and dexamethasone; SOC, standard of care.
*Adapted from Einsele.5
Results
The intent-to-treat population included 419 patients, 211 in the SOC arm and 208 in the cilta-cel arm. Overall, 208 patients received SOC therapy and 176 ultimately received cilta-cel; 32 were excluded due to progression during bridging therapy. Table 1 outlines the baseline patient characteristics of the intent-to-treat population.5
Table 1. Baseline characteristics of the ITT population*
|
cilta-cel, ciltacabtagene autoleucel; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ISS, International Staging System; PI, proteasome inhibitor; SOC, standard of care. |
||
|
Characteristic, % (unless otherwise stated) |
ITT Population |
|
|---|---|---|
|
Cilta-cel |
SOC |
|
|
Median age (range), years |
61.5 (27–78) |
61.0 (35–80) |
|
Male |
55.8 |
58.8 |
|
Race† |
|
|
|
Asian |
7.7 |
9.5 |
|
Black |
2.9 |
3.3 |
|
White |
75.5 |
74.4 |
|
Other |
0.5 |
0.5 |
|
ISS stage |
|
|
|
I |
65.4 |
62.6 |
|
II |
28.8 |
30.8 |
|
III |
5.8 |
6.6 |
|
Bone marrow plasma cells ≥60% |
20.4 |
20.7 |
|
Presence of soft-tissue plasmacytomas |
21.2 |
16.6 |
|
Prior lines of therapy |
|
|
|
1 |
32.7 |
32.2 |
|
2 |
39.9 |
41.2 |
|
3 |
27.4 |
26.5 |
|
Cytogenetic high-risk‡ |
59.4 |
62.9 |
|
del(17p) |
23.7 |
20.5 |
|
t(14;16) |
1.4 |
3.3 |
|
t(4;14) |
14.5 |
14.3 |
|
gain/amp(1q) |
43.0 |
51.0 |
|
2 or more high-risk |
20.8 |
23.3 |
|
del(17p), t(14;16) or |
35.3 |
32.9 |
|
Triple-class exposed |
25.5 |
26.1 |
|
Refractory status |
|
|
|
Triple-class refractory |
14.4 |
15.6 |
|
Any PI |
49.5 |
45.5 |
|
Bortezomib |
26.4 |
22.7 |
|
Pomalidomide |
3.8 |
4.3 |
|
Daratumumab |
23.1 |
21.3 |
Efficacy
At follow up of 15.9 months, the median duration of progression-free survival was not reached in the cilta-cel group and 11.8 months in the SOC group. The progression-free survival rate at 12 months in the intent-to-treat populations is highlighted in Figure 2.5
Figure 2. 12-month PFS rate for cilta-cel vs SOC in the intent-to-treat population*†

cilta-cel, ciltacabtagene autoleucel; PFS, progression-free survival; SOC, standard of care.
*Data from San-Miguel, et al.1
†SOC denotes pomalidomide, bortezomib, and dexamethasone or daratumumab, pomalidomide, and dexamethasone.
Secondary endpoints included CR, overall response rate, OS, and safety. Patients in the cilta-cel arm achieved higher complete or stringent-complete response rates, with 73.1% achieving CR or better in the cilta-cel arm versus 21.8% in the SOC arm. However, the SOC arm achieved higher levels of stable disease (22.3% vs 6.2%) and lower rates of progressive disease than the cilta-cel arm.1 A summary of the secondary endpoint data is shown in Figure 3.
Figure 3. Treatment responses in the intent-to-treat population*

cilta-cel, ciltacabtagene autoleucel; CR, complete response; PR, partial response; sCR, stringent complete response; SOC, standard of care; VGPR, very good partial response.
*Data from San-Miguel, et al.1
Safety
The safety population, which including patients who initiated treatment in both cohorts, initially consisted of 208 patients in each arm. All patients experienced at least one adverse event (AE) of any grade. Patients receiving cilta-cel experienced more Grade 3 or 4 AEs (96.6% versus 94.2%), with the most common AEs being hematologic in both populations. The most commonly occurring non-CAR T-related AEs in the safety populations are outlined in Figure 4.1
Figure 4. Most common AEs in the safety populations*
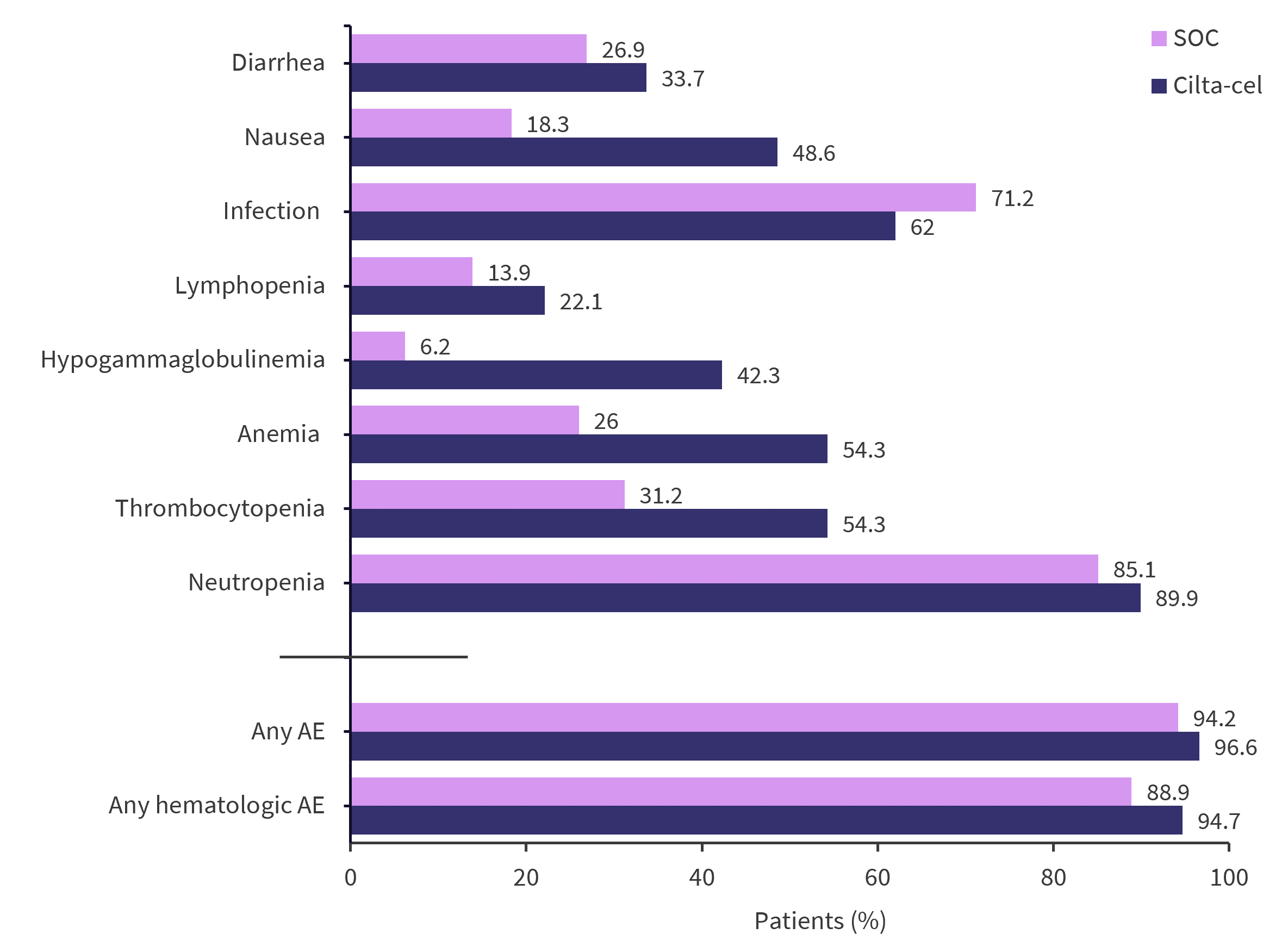
AE, adverse event; cilta-cel, ciltacabtagene autoleucel; SOC, standard of care.
*Data from San-Miguel, et al.1
Of the 176 patients who ultimately received cilta-cel, 76.1% experienced CAR T-associated cytokine release syndrome, with 1.1% being Grade 3 or 4. Also, 20.5% experienced any grade neurotoxicity, with immune effector cell–associated neurotoxicity syndrome occurring in 4.5%.1 CAR T-cell-related adverse events in the cilta-cel population (n = 176) are shown in Figure 5.
Figure 5. CAR T-cell-associated adverse events in the cilta-cel cohort (n = 176)*

ICANS, Immune effector cell–associated neurotoxicity syndrome.
*Data from San-Miguel, et al.1
Conclusion
Single infusion cilta-cel in patients with lenalidomide-refractory MM resulted in higher rates of progression-free survival and a lower incidence of mortality at 15.9-month follow up compared with SOC. Rates of complete response or better were also greater in the cilta-cel cohort; however, there was a slightly increased incidence of progressive disease. Cilta-cel may improve clinical outcomes in patients, although a higher incidence of adverse events and side effects has also been reported.
Furthermore, CAR T-cell therapies are not appropriate for all patients with relapsed/refractory MM. For example, current contraindications for treatment include an ongoing infection and a high tumor burden.6 Though longer follow up is needed to fully elucidate survival outcomes, the CARTITUDE-4 and the KarMMa-3 trial (NCT03651128) represent the first phase 3 trials with positive interim results comparing a BCMA-directed CAR T-cell therapy to the current standard of care in earlier lines of therapy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



