All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Infection incidence with CAR T-cell and bispecific antibody therapies
Do you know... Bacterial infections are common in the period after CAR T-cell therapy. What percentage of infections are reported as bacterial among patient cohorts in most studies?
Infection is a common adverse event during chimeric antigen receptor (CAR) T-cell and bispecific antibody therapies, with the extent, type, and timing of risk dictated by several factors.1 Baseline infection may vary depending on the type of myeloma and treatment regimens used, and reporting of infections during therapy is often insufficiently detailed to describe the epidemiology. Furthermore, there is a lack of standardization between microbiologically versus clinically-defined infections and prophylaxis is often varied and poorly described. Together, these issues present challenges in understanding the incidences associated with CAR T-cell and bispecific antibody therapies.1
To bridge this knowledge gap, Hammond gave a presentation at the 4th Immune Effector Cell Therapies in Multiple Myeloma (MM) Workshop on infection incidence associated with CAR T-cell and bispecific antibody therapy. We summarize the key points in the article below.
Late infections
CAR T-cell therapy is commonly associated with a risk of infection and especially infections that present after an extended period post infusion:
- Infection risk appears to decline quickly after the first 30–100 days following CAR T therapy.
- Despite differences in estimated infection rates, many current studies suggest that bacteria are the dominant cause of infection, accounting for 60–80% of all infections during the first 100 days after treatment.
- After this 100-day period, infections are more likely to be viral.
- This was highlighted by the Fred Hutchinson Cancer Research Center 17 trial,2 in which 53% of the 32-patient population developed 23 infections during the first 180 days, 13 of which were viral.
- This was a similar case in the Dana Faber Cancer Center/Mass General Hospital cohort of 99 patients,3 where they experienced a 46% increase in respiratory infections including pneumonia, sinusitis, and upper/lower respiratory tract infections.
Long-term risk factors and hypogammaglobulinemia
Several studies have highlighted potential risk factors associated with an increased infection risk post infusion. These include cases of hypogammaglobulinemia and the number of previous lines of therapy:
- In a retrospective analysis of a patient cohort based at the University of California San Francisco,4 53% of 55 patients experienced infection within the first year, of which 25 cases were viral.
- Among these patients, hypogammaglobulinemia was common and persistent throughout (Figure 1).
- In a retrospective review of all patients treated with CAR T therapy at the Mount Sinai Hospital,5 95% of 80 patients developed hypogammaglobulinemia over a median of 30.1 months.
- Patients treated with 1–3 lines of therapy had a lower rate of infection compared with those treated with ≥4 lines of therapy.
Figure 1. Changes in IgG levels in a patient cohort during the first 12 months after CAR T-cell therapy*
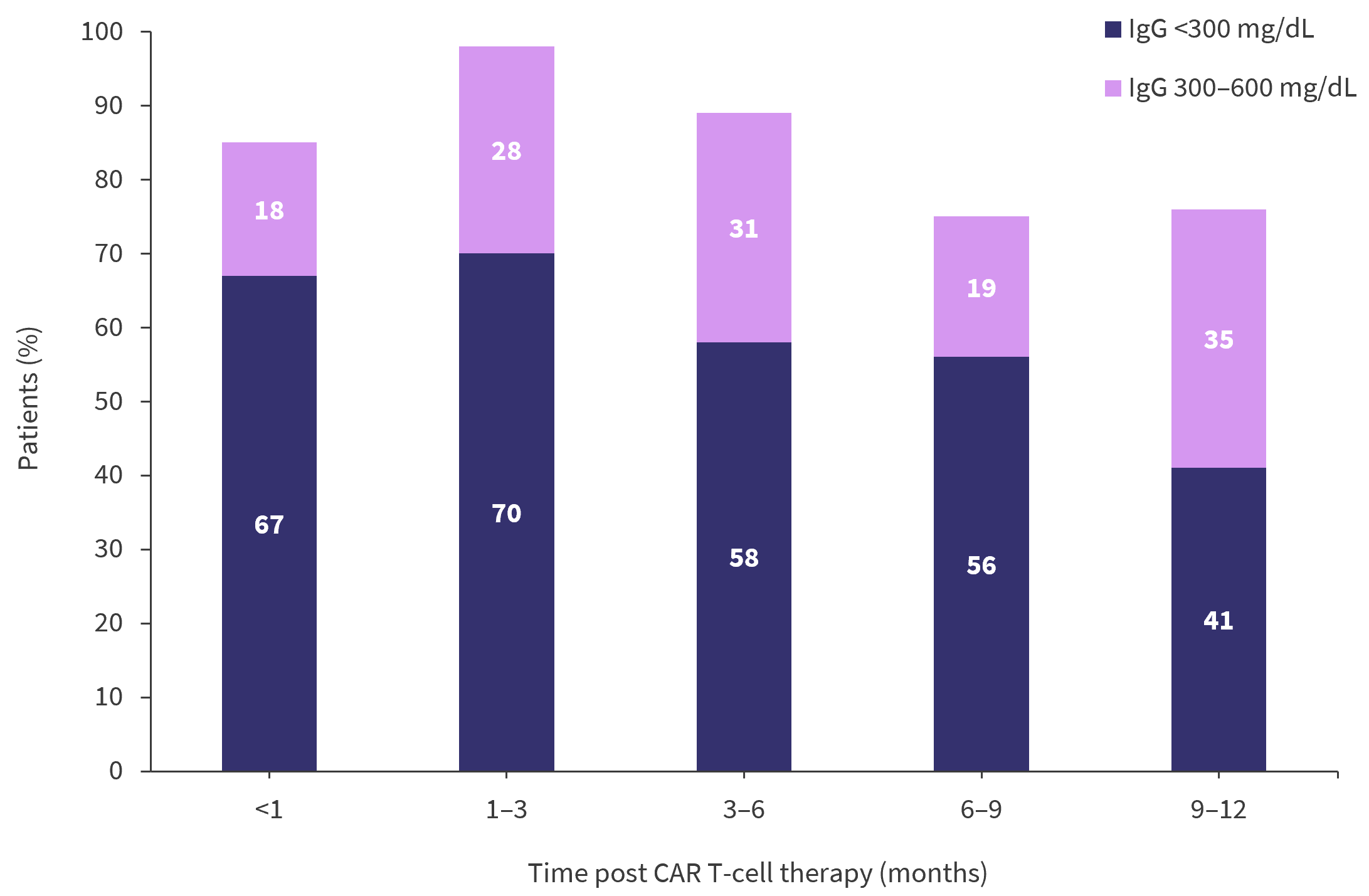
CAR, chimeric antigen receptor; IgG, immunoglobulin G.
*Adapted from Hammond1 and Kambhampati, et al.4
Viral reactivation risk
Cytomegalovirus (CMV) reactivation events have been reported among patients treated with CAR T-cell therapy. Reports have included:
- A case of CMV retinitis in a patient who was in remission 3 months after treatment from a study published by Zu et al.6
- Six events of viral reactivation across 61 patients at a median of 66 days from a study by Wang et al.7
- Reactivation in 34% of patients from an 80-patient cohort at a median of 30.1 months from a study performed by Lancman et al.8
It remains unclear whether these rates are higher than those normally associated with patients diagnosed with relapsed/refractory MM as both hematopoietic stem cell transplantation and daratumumab treatment have also been associated with CMV reactivation.
Infection after bispecific antibody therapy
Early studies have suggested that infection after bispecific antibody treatment may be even more common than infection associated with CAR T-cell therapy and that infection rates vary depending on the antibody target.
- A recent review of 1,185 patients from eleven different trials found an overall rate of infection of 50%, with a median of 6.1 months of follow up.
- In comparison, non-B-cell maturation antigen (BCMA) bispecific antibody therapy was associated with significantly less neutropenia and Grade 3/4 infections versus BCMA bispecific antibody therapy (Figure 2).
- Across recent clinical trials, COVID-19 accounted for a large proportion of infections.
- 7% of patients receiving talquetamab in the MonumenTAL-1 study (NCT04634552).
- 6–18% of patients enrolled in the trials investigating ABBV-383, elranatamab, and teclistamab.
Figure 2. Rates of neutropenia and infection in patients treated with BCMA and non-BCMA antibody therapy*

BCMA, B-cell maturation antigen.
*Adapted from Hammond.1
Opportunistic infections
Opportunistic infections have also been reported across several trials. Pneumocystis was reported in 3.6% of patients enrolled in a phase I/II trial of teclistamab. Fatal adenovirus pneumonia was also reported in this trial, along with multiple cases in other trials and studies, including a phase I trial of talquetamab. Other opportunistic infections reported included:
- BK virus
- Salmonellosis
- Prolonged symptomatic norovirus infection
- Disseminated varicella-zoster virus and ophthalmic herpes simplex virus
Conclusion
While CAR T-cell and bispecific antibody therapies offer important treatment opportunities for patients with MM, late and opportunistic infections after treatment present significant challenges to achieving optimal patient outcomes. Bacterial and viral infections are common, and hypogammaglobulinemia leading to a risk of viral reactivation and severe opportunistic infections is widespread and persistent. A focus on appropriate prophylactic and management techniques is essential for improving patient outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
HCPs, how confident do you feel discussing ocular care and potential concerns associated with belantamab mafodotin with your patients?

