All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Long-term outcomes following CAR T-cell therapy in B-cell malignancies
Do you know... In which of the following hematologic malignancies have CD19-targeted CAR T-cell therapies demonstrated curative potential in a subset of patients?
CD19-directed chimeric antigen receptor (CAR) T-cell therapies demonstrated potent activity in early phase trials of patients with relapsed/refractory (R/R) B-cell malignancies and further achieved high complete remission (CR) rates in subsequent multicentre trials. More recently, B-cell maturation antigen (BCMA)-directed CAR T-cell therapies yielded high overall response rates (ORR) in patients with R/R multiple myeloma (RRMM).1
These improved outcomes resulted in the U.S. Food and Drug Administration (FDA) approval of four CD19-targeted CAR T-cell therapies: axicabtagene ciloleucel (axi-cel), lisocabtagene maraleucel (liso-cel), tisagenlecleucel (tisa-cel), and brexucabtagene autoleucel (brexu-cel) for R/R B-cell lymphomas; the latter two are indicated in B-cell acute lymphoblastic leukemia (ALL). Two B-cell maturation antigen (BCMA)-targeted CAR T-cell therapies, idecabtagene vicleucel and cilta-cabtagene autoleucel (cilta-cel), have been FDA approved for RRMM. These CAR T-cell products share similar adverse effects with variable differences.1
Although CAR T-cell therapy has significantly advanced the treatment landscape of B-cell malignancies, its labor-intensive process and cost warrants further understanding of its long-term outcomes.
Below, we summarize the review article published by Cappell et al.1 in Nature Reviews Clinical Oncology on long-term outcomes following CAR T-cell therapy in B-cell malignancies, including data on efficacy and safety, factors associated with long-term remissions, and ongoing investigational strategies to improve durable remissions.
FDA-approved CAR T-cell products in B-cell malignancies
Below, we provide an overview of the treatment indications for all current FDA-approved CAR T-cell therapies in R/R B-cell lymphoma, B-ALL, and MM (Figure 1).
Figure 1. FDA-approved CAR T-cell therapies*
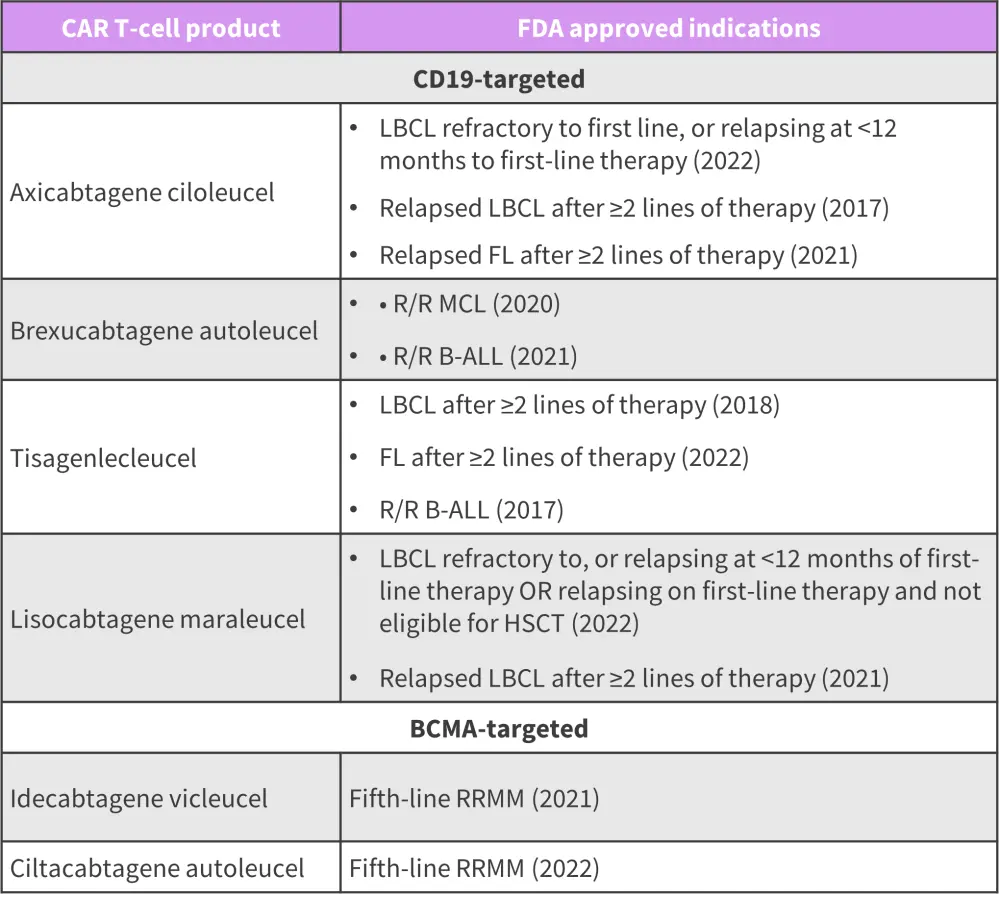
B-ALL, B-cell acute lymphoblastic leukemia; CAR, chimeric antigen receptor; FDA, Food and Drug Administration; FL, follicular lymphoma; HSCT, hematopoietic stem cell transplantation; LBCL, large B-cell lymphoma; MCL, mantle cell lymphoma; R/R, relapsed/refractory; RRMM, relapsed refractory multiple myeloma.
*Adapted from Cappell, et al.1
Long-term efficacy outcomes1
CD19 CAR T-cell therapy in B-cell lymphomas and CLL
Data from ten studies assessing CD19 CAR T cells in R/R B-cell lymphomas, chronic lymphocytic leukemia (CLL) and small lymphocytic leukemia have provided ≥24 months follow-up data (Figure 2). At ≥2 years after CAR T-cell infusion, durable responses were observed in a subset of patients across all studies, CAR T-cell products, and malignancies. Overall, data suggest the curative potential of CAR T-cell therapy for some patients with R/R B-cell lymphomas.
Figure 2. Long-term efficacy in B-cell lymphomas and/or CLL/SLL*
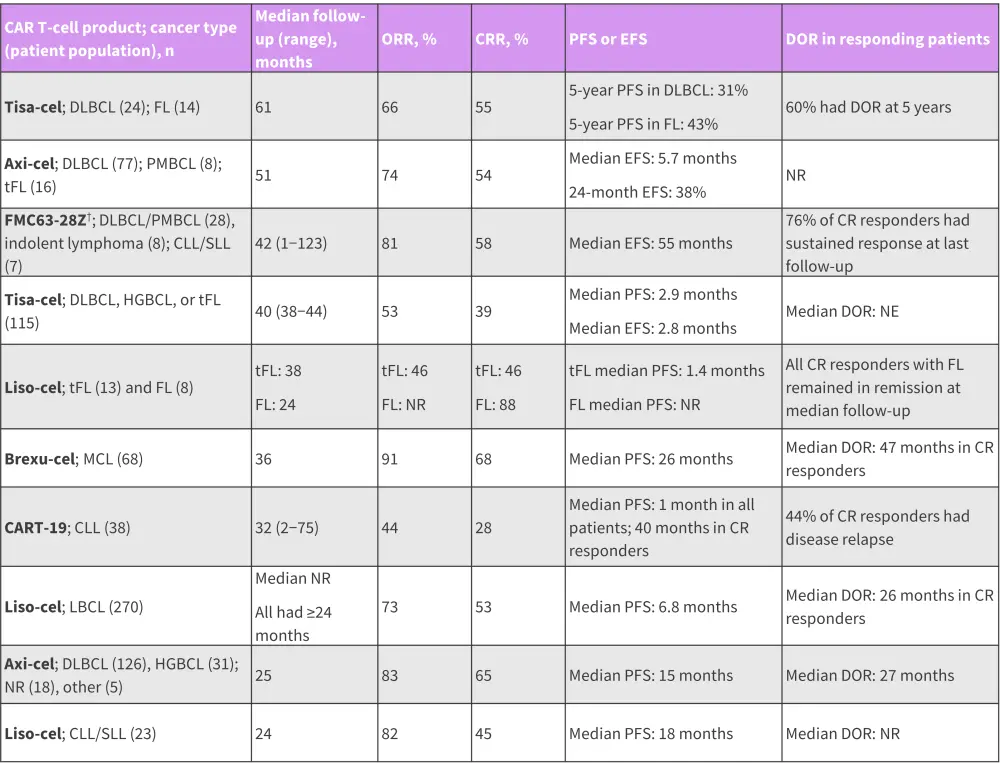
axi-cel, axicabtagene ciloleucel; CAR, chimeric antigen receptor; CRR, complete remission rate; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; DOR, duration of response; EFS, event free survival; FL, follicular lymphoma; HGBCL, high-grade B-cell lymphoma; liso-cel, lisocabtagene maraleucel; MCL, mantle cell lymphoma; NR, not reported; PFS, progression-free survival; PMBCL, primary mediastinal large B-cell lymphoma; SLL, small lymphocytic leukemia; tFL, transformed follicular lymphoma; tisa-cel, tisagenlecleucel.
*Data from Cappell, et al.1
†LCAR-B38M was later developed to cilta-cabtagene autoleucel and FMC63-28Z to axicabtagene ciloleucel.
CD19 CAR T-cell therapy in B-ALL
Data from 12 studies at a median follow-up of 1-year (range, 1–4.8 years) outline the long-term efficacy of CD19-targeted CAR T-cell therapies in B-ALL.
- High CR rates of 62–86% have been achieved, most of which are minimal residual disease (MRD)-negative remissions.
- Variable median event-free survival (EFS; median EFS, 3.1−24 months) rates reported with a substantial but variable number of patients proceeding to allogeneic hematopoietic stem cell transplantation (allo-HSCT; 17−88%) in remission.
- Long-term studies (>24 months) on tisa-cel and brexu-cel in adult patients reported a CR of 69%, with a median EFS and relapse-free survival (RFS) of 5.6 and 7 months, respectively.
- Long-term data from the ELIANA trial (NCT02435849) of tisa-cel in younger patients (<25 years of age) yielded a CR rate of 82% and median EFS of 24 months; this indicates higher survival outcomes in pediatric vs adult populations.
- The 3-year RFS for patients in ELIANA undergoing alloHSCT vs non allo-HSCT was 52% vs 48%, respectively; this highlights that a proportion of pediatric patients can achieve long-term remissions without allo-HSCT consolidation.
- Data on other CD19 CAR T-cell products in pediatric patients without allo-HSCT showed a relapse rate of 68–100%, demonstrating that long-term remissions can differ between CAR T-cell products in the pediatric population.
- Allo-HSCT is recommended in adults due to lower median EFS outcomes compared with pediatrics; data show an improved EFS for those receiving allo-HSCT after tisa-cel.
- Important factors to identify patients who could benefit from allo-HSCT include receipt of previous HSCT, loss of B-cell aplasia, previous treatments received, cytogenetics, MRD detection, and disease burden prior to CAR T-cell infusion.
- Overall, CD19-targeted CAR T-cell therapy can induce high CR rates in patients with B-ALL; however, its lower survival rates compared to B-cell lymphomas indicate a lack of curative potential without subsequent therapy in B-ALL.
BCMA-directed CAR T-cell therapy in RRMM
Compared with other B-cell malignancies, there is less data on the long-term outcomes following BCMA-directed therapies in RRMM. Here, we report data from six studies at a median follow-up of ≥1 year (range, 13–48 months).
- ORR of 73–100% and CRs of 33−83% with frequent MRD-negative remissions reported
- Long-term remissions observed in a subset of patients across all studies without the use of consolidative or maintenance therapy
- Median PFS varied significantly across studies, ranging from 5.2 to 27 months
- Longest follow-up data on idecabtagene vicleucel at a median of 13 months reported median PFS of 8.8 months; 33% patients achieving CR had a median DOR of 19 months
- Long-term follow-up data at a median of 28 months on cilta-cel in the US and Japan reported a PFS of 55% at 27 months, although median DOR was not estimable and median PFS not reached
- Another long-term study on cilta-cel at median follow-up of 48 months in patients based in China showed a median PFS of 18 months
- Data show that BCMA-targeted therapies can achieve durable remissions in patients with RRMM, though there is a risk of progression over time
Factors associated with long-term remissions1
Several factors have been linked with durable remissions following CAR T-cell therapy, including depth of response, type and characteristics of malignancy, tumor burden and location, lymphodepletion chemotherapy, and CAR T-cell levels. Figure 3 summarizes the clinical data for each factor.
Figure 3. Factors associated with long-term remissions*
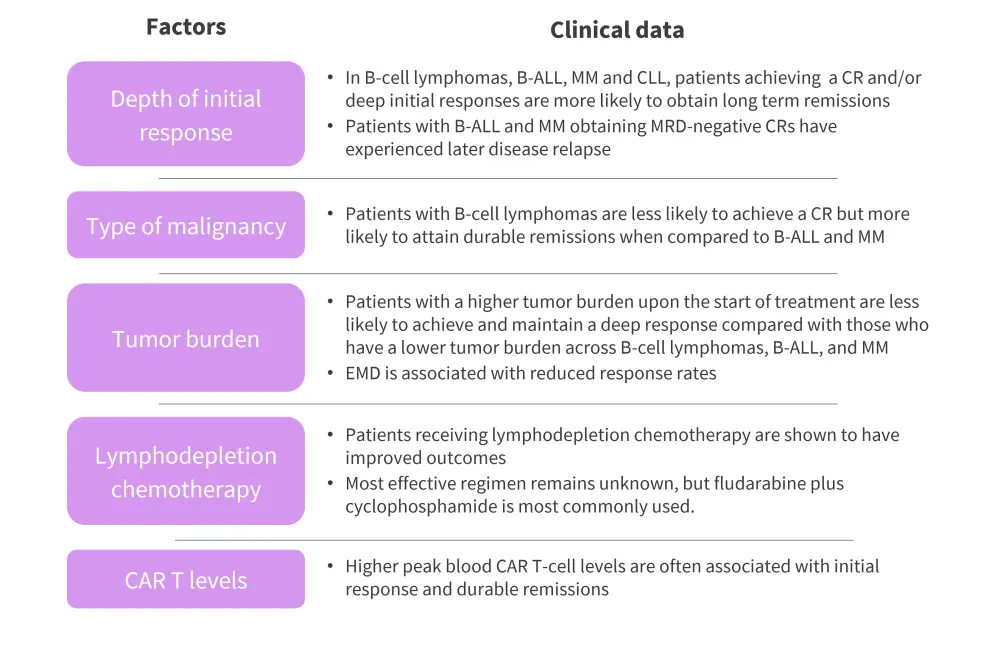
B-ALL, B-cell acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; CR, complete response; EMD, extramedullary disease; MM, multiple myeloma; MRD, minimal residual disease
*Adapted from Cappell, et al.1
Long-term adverse effects1
Although there is limited data regarding long-term adverse effects following CAR T-cell therapy, the most commonly observed toxicities thus far include B-cell depletion, hypogammaglobulinemia, cytopenias, and infections. Risk factors associated with cytopenias include higher-grade cytokine release syndrome, multiple prior lines of therapy, allo-HSCT ≤1 year prior to CAR T-cell infusion, baseline cytopenia, and the presence of bone marrow malignancy. Available data indicate an increased risk of infections; however, there is no evidence so far on the long-term risk of secondary malignancies post CAR T-cell infusion. Table 1 reports long-term safety data occurring ≥90 days post CAR T-cell infusion across MM, B-ALL, and B-cell lymphomas.
Table 1. Long-term toxicities in CAR T-cell therapies*
|
ALL, acute lymphoblastic leukemia; axi-cel, axicabtagene ciloleucel; CAR, chimeric antigen receptor; CR, complete remission; CLL, chronic lymphocytic leukemia; IgG, immunoglobulin; liso-cel, lisocabtagene maraleucel; MDS, myelodysplastic syndromes; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; NR, not reported; tisa-cel, tisagenlecleucel. |
|||||
|
CAR T-cell product |
Median follow-up (range), months |
Prevalence of persistent B-cell/IgG depletion in CR, % |
Prevalence of severe cytopenias, % |
Incidence of late infections, % |
Incidence of second malignancy, % |
|---|---|---|---|---|---|
|
Tisa-cel (adults with B-cell lymphoma; n = 38) |
61 |
B-cell: 33 |
3 |
NR |
16 |
|
LCAR-B38M† (adults with MM; n = 74) |
48 |
NR |
NR |
NR |
5 |
|
FMC63-28Z† (adults with B-cell lymphoma or CLL; n = 43) |
42 (1−123) |
B-cell: 38 |
NR |
9; requiring hospitalization >6 months post infusion |
16 |
|
Liso-cel (adults with ALL, NHL, or CLL; n = 86) |
28 (13−63) |
B-cell: NR |
16 in CR |
61; 80% non-severe and 20% requiring hospitalization >3 months post infusion |
15 |
|
Axi-cel (adults with B-cell lymphomas; n = 108) |
27 (26−29) |
B-cell: 25 |
17 |
2 Grade 3 infections at >12 months post infusion in those with ongoing remission |
1 case of MDS |
Ongoing investigational strategies in CAR T-cell therapy
There are ongoing investigational strategies to improve outcomes following CAR T-cell therapy by optimizing all areas of the process, including patient selection, pre- and post-CAR T-cell infusion treatment, and cell manufacturing (Figure 4).
Figure 4. Investigational strategies to improve CAR T-cell therapy*
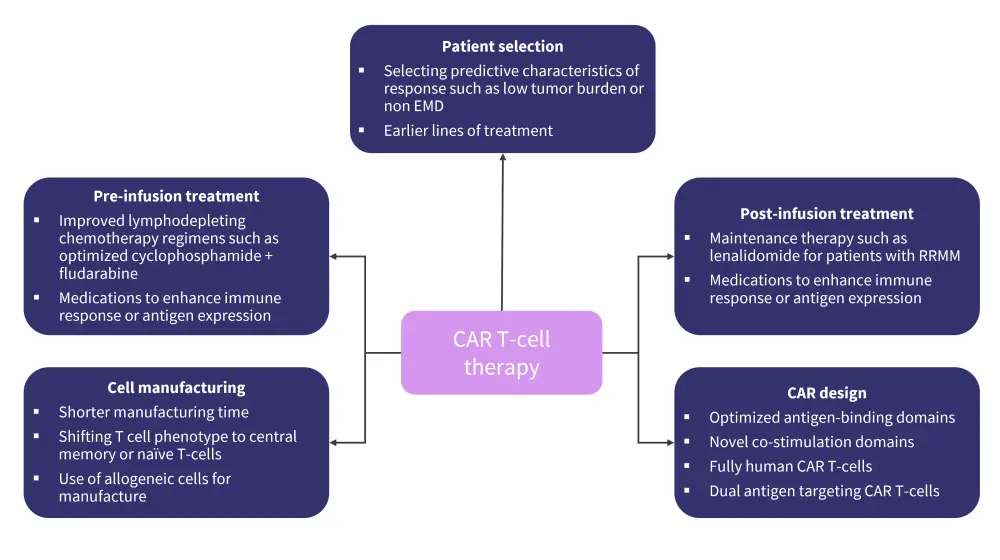
CAR, chimeric antigen receptor; EMD, extramedullary disease; RRMM, relapsed refractory multiple myeloma.
*Adapted from Cappell, et al.1
Given that antigen escape is an established relapse mechanism in CAR T-cell therapy, strategies such as dual antigen targeted CAR T-cells are being actively studied. We have previously reported key data from three investigational studies assessing dual-targeted CAR T-cell therapies in DLBCL, MCL, and Richter’s transformation, which demonstrated promising activity. Long-term analyses in B-cell lymphomas and B-ALL have also reported disease relapse due to antigen loss, suggesting that a combination strategy could prove beneficial in overcoming resistance.
Fully human CAR T-cell products have demonstrated CAR T-cell persistence but have not yet demonstrated improved efficacy. Development of substituted single-chain with heavy-chain variable domains such as cilta-cel have demonstrated high efficacy; however, prospective analyses are needed.
Axi-cel as a first-line therapy in high-risk B-cell lymphomas has been investigated in the ZUMA-12 trial (NCT03761056), demonstrating CR rates of 78%, 86% of which were durable. Other ongoing trials assessing CAR T-cells in earlier lines include axi-cel versus standard of care in first-line high-risk B-cell lymphoma (ZUMA-23; NCT05605899); and cilta-cel in newly diagnosed MM (CARTITUDE-5; NCT04923893). Emerging data have indicated that γ-secretase inhibitors before BCMA-targeted CAR T-cell therapy, ibrutinib prior to CD19-targeted CAR T-cells, and immune-checkpoint inhibitors after CAR T cells could be viable options to alter antigen expression and/or CAR T-cell function.
Conclusion
Overall, long-term data demonstrate the high efficacy and minimal levels of toxicity associated with BCMA/CD19-targeted CAR T-cell therapy in hematological malignancies. While CAR T-cells induce durable remissions and have curative potential in B-cell lymphomas, it remains an important bridging therapy to allo-HSCT for durable efficacy in B-ALL. CAR T-cells can attain prolonged remission in MM, though its curative potential is not yet established. The ongoing research efforts in CAR T-cell development are likely to further improve durable remissions and expand its treatment indications.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

