All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Ixazomib, lenalidomide, and dexamethasone as first-line therapy: Tourmaline-MM2 trial update
Visual abstract
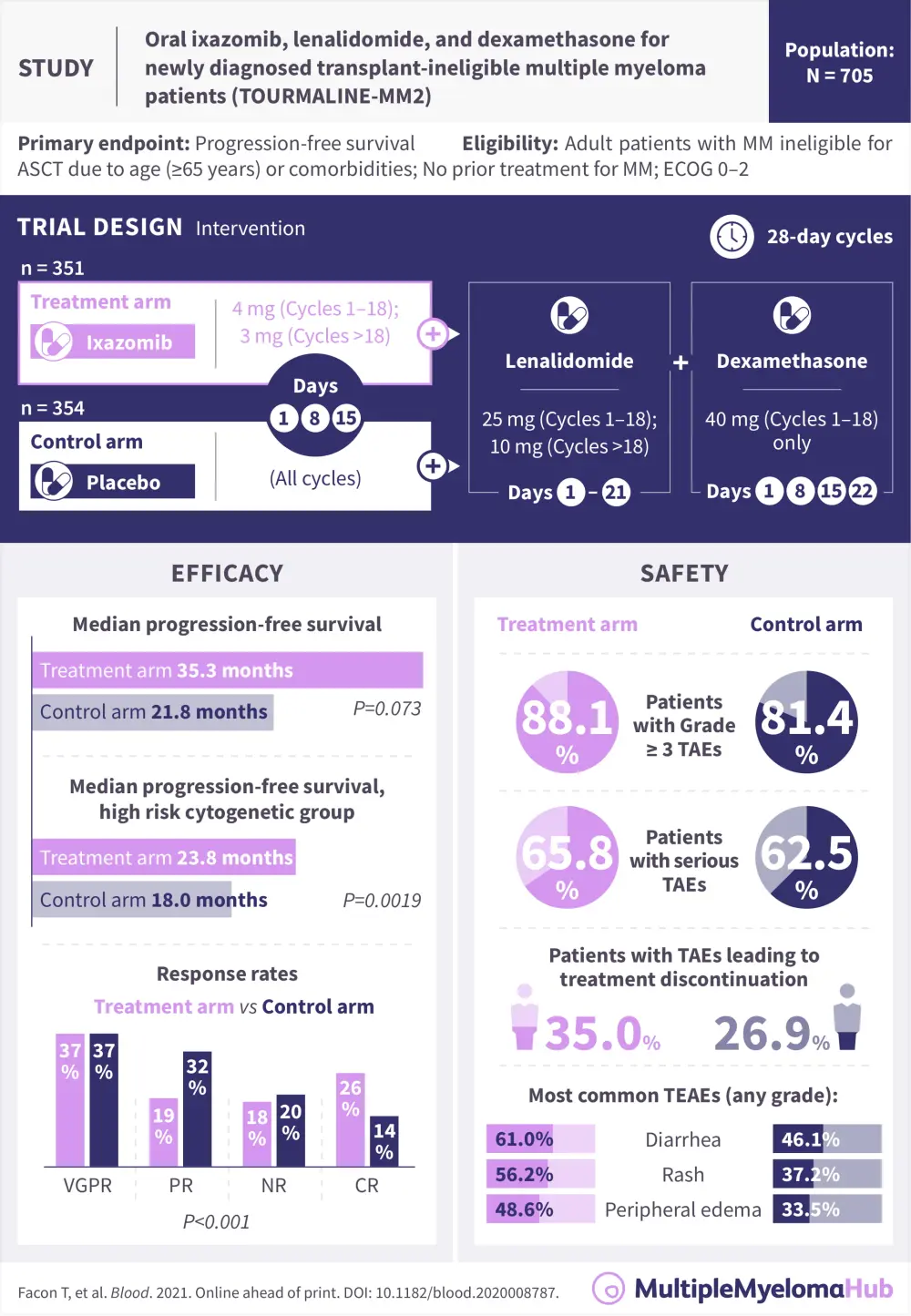
Most treatment options for newly diagnosed patients with multiple myeloma (NDMM) who are ineligible for transplant involve lenalidomide and dexamethasone (Rd-)based regimens. Rd in combination with proteasome inhibitors (PIs) have demonstrated beneficial results in survival outcomes; however, long-term use of injectable PI agents can be challenging for some patients. An all-oral triplet combination would reduce the need for patients to travel to the clinic as often and reduce the disease burden.
In a recent article in Blood, Thierry Facon and colleagues discussed the results of the Tourmaline-MM2 trial (NCT01850524), which tested the triplet combination of ixazomib-Rd against placebo-Rd in transplant-ineligible patients with NDMM.1
The Multiple Myeloma Hub previously provided a detailed summary of the results of the Tourmaline-MM2 trial, here. This report provides a short update.
Measurable residual disease (MRD) and survival outcomes
Further results were reported with respect to MRD in this update. In the placebo-Rd arm, 17.5% of patients had a bone marrow aspirate taken compared with 28.8% of patients in the ixazomib-Rd arm. These samples were used for MRD evaluation by flow cytometry with a sensitivity of 10–5. Of these patients, it was found that:
- 52.5% of patients in the ixazomib-Rd arm were MRD negative.
- 38.7% of patients in the placebo-Rd arm were MRD negative.
In the intention-to-treat population, this resulted in MRD negative rates of:
- 15.1% in the ixazomib-Rd arm.
- 6.8% in the placebo-Rd arm.
Median overall survival was not reached in either arm. Time to progression and time to response were significantly improved in the treatment arm compared with the placebo arm. Duration of response was also increased from 37.5 in the placebo-Rd arm to 50.6 months in the ixazomib-Rd arm (Table 1).
Table 1. Survival outcomes between treatment arms*
|
d, dexamethasone; R, lenalidomide. |
|||
|
|
Ixazomib-Rd (n = 351) |
Placebo-Rd (n = 354) |
p value |
|---|---|---|---|
|
Time to progression, months |
45.8 |
26.8 |
p = 0.008 |
|
Time to response, months |
1.0 |
1.9 |
p < 0.001 |
|
Duration of response, months |
50.6 |
37.5 |
— |
Conclusion
Ixazomib-Rd was demonstrated to be an effective treatment for transplant-ineligible patients with NDMM, with 15.1% of patients in the treatment arm reaching MRD-negativity at 10–5. For patients with reduced mobility who find daily travel to the clinic difficult, this all-oral triplet combination could be a preferable treatment option.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

