All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Initial results of daratumumab + lenalidomide in frail patients: The IFM 2017-03 trial
The International Myeloma Foundation (IFM) 2017-03 (IFM 2017-03) trial (NCT03993912) is a phase III clinical trial exploring the use of daratumumab and lenalidomide (DR) compared with lenalidomide and dexamethasone (Rd) for the treatment of patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant.1
The Multiple Myeloma Hub is pleased to summarize the oral presentation given by Manier1 at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, which detailed outcome and safety data from the IFM 2017-03 trial. Their research question was: does the benefit of dexamethasone justify potentially higher rates of infection and pneumonia in frail patients?1
Study design
The IFM 2017-03 trial randomized patients to Rd versus DR in the ratio 1:2. All patients had NDMM, were ≥65 years, and had an IFM frailty score of ≥2 (calculated using age, Eastern Cooperative Oncology Score, and Charlson Comorbidity Index).2 The study design can be seen in Figure 1. Treatment in both arms was given until disease progression or unacceptable toxicity, with low dose dexamethasone (20 mg/week) in Arm B.
Figure 1. Study design of the IFM 2017-03 trial*
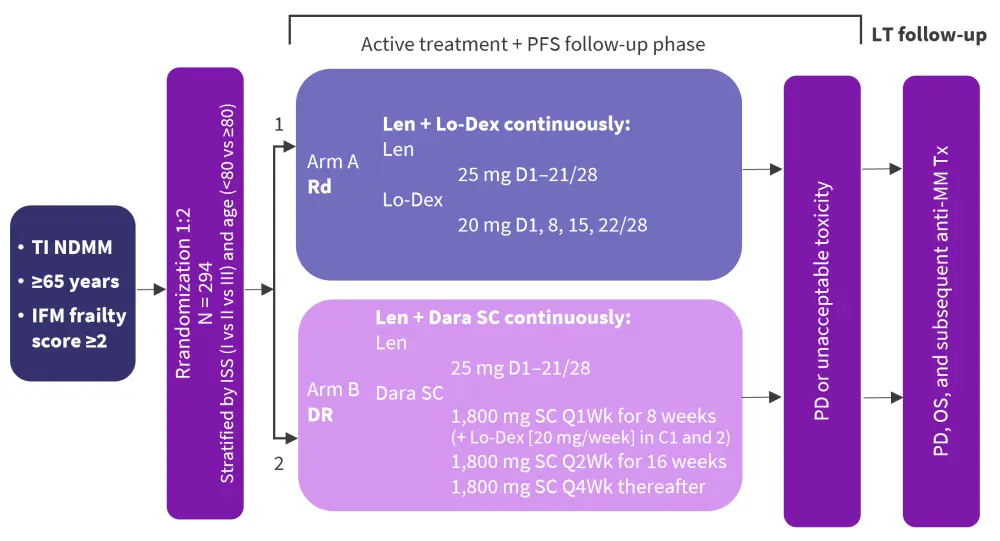
C, Cycle; Dara, daratumumab; Dex, dexamethasone; DR, lenalidomide and dexamethasone; IFM, International Myeloma Foundation; Len, lenalidomide; Lo-Dex, low-dose dexamethasone; LT, long-term; NDMM, newly diagnosed multiple myeloma; OS, overall survival; PD, progressive disease; Rd, lenalidomide and low-dose dexamethasone; SC, subcutaneous.
*Adapted from Manier.1
This pre-planned interim analysis included overall response rate, very good partial response or better (≥VGPR), minimal residual disease (MRD) rate, and the occurrence of ≥Grade 3 adverse events. Longer follow-up is ongoing to evaluate progression-free survival assessed up to 84 months (primary endpoint), as well as time-to-treatment failure, time-to-next treatment, and overall survival, among other secondary outcomes.
Results
In total, 295 patients were recruited, with 199 in the DR arm compared with 94 in the Rd arm. Patient disposition can be seen in Figure 2. In total, 3 patients dropped out prior to treatment. For the analysis presented, data cut off was performed at 12 months, with a higher percentage of Rd patients discontinuing treatment compared with DR patients (Figure 2).
Figure 2. Patient disposition of patients recruited to IFM 2017-03*
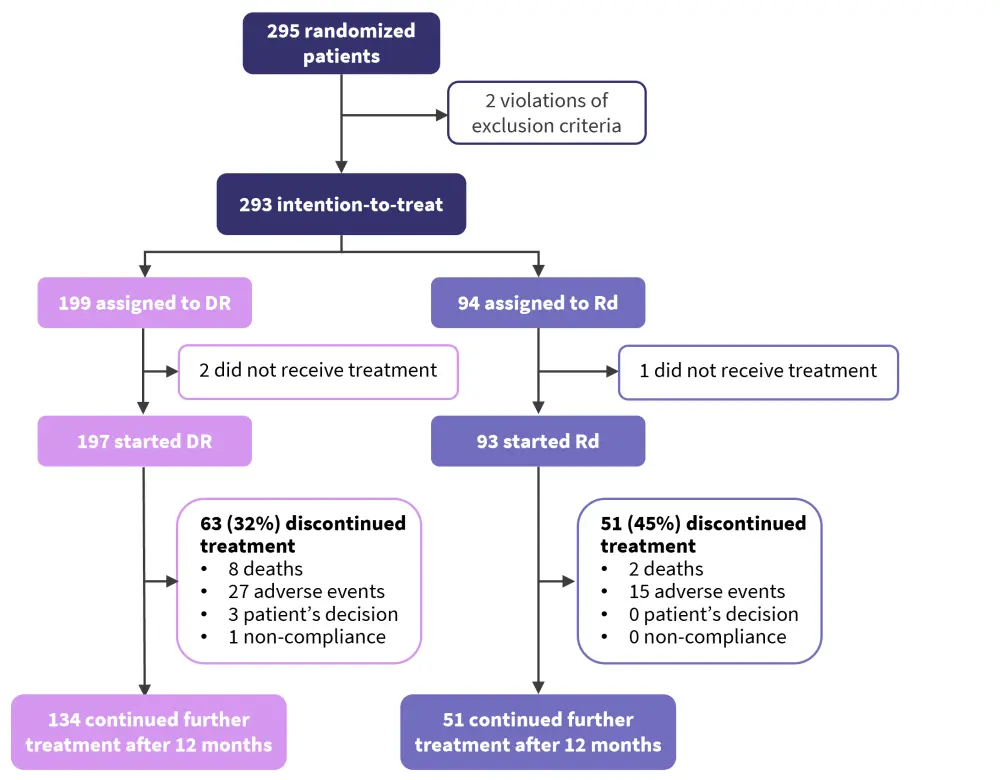
DR, lenalidomide and daratumumab; IFM; International Myeloma Foundation; Rd, lenalidomide and dexamethasone.
*Adapted from Manier.1
Patient demographics can be seen in Table 1, showing that patients in both groups were well matched for age and frailty.
Table 1. Patient data from the IFM 2017-03 trial*
|
DR, lenalidomide and dexamethasone; ECOG, Eastern Cooperative Oncology Group; IFM, International Myeloma Foundation; ISS, International Staging System; Rd, lenalidomide and dexamethasone. |
||
|
Characteristic, % |
Patients treated with DR |
Patients treated with Rd |
|---|---|---|
|
Age ≥80 years |
59 |
65 |
|
Sex |
|
|
|
Female |
51 |
51 |
|
Male |
49 |
49 |
|
ECOG |
|
|
|
0 |
10 |
10 |
|
1 |
46 |
50 |
|
2 |
44 |
40 |
|
IFM frailty score |
|
|
|
≤1 |
0 |
0 |
|
2 |
29 |
37 |
|
3 |
41 |
28 |
|
4 |
22 |
26 |
|
5 |
9 |
10 |
|
ISS disease stage |
|
|
|
I |
17 |
19 |
|
II |
51 |
53 |
|
III |
32 |
28 |
|
Cytogenetic risk profile |
|
|
|
Standard |
83 |
78 |
|
High |
17 |
22 |
The analysis presented was from a data cut-off of 12 months:
- Overall response rate was 96% in the DR group compared with 85% in the Rd group (p = 0.001), with a ≥VGPR rate of 64% vs 43%, respectively, that improved over time.
- MRD assessment by next generation sequencing at 10−5 was performed in patients with ≥VGPR at 12 months and patients with missing MRD data were considered positive. At a 10−5 sensitivity in an intention-to-treat interim analysis, DR significantly improved rates of MRD negativity compared with Rd (10% vs 3%; p = 0.012).
- Safety data can be seen in Table 2. Treatment discontinuation due to adverse events for DR and Rd were 14% and 16%, respectively, with no statistically significant difference.
Table 2. Grade ≥3 adverse events in the IFM 2017-03 trial*
|
AE, adverse event; DR, lenalidomide and dexamethasone; IFM, International Myeloma Foundation; Rd, lenalidomide and dexamethasone; SAE, serious adverse event. |
|||
|
Characteristic, % |
DR group |
Rd group |
p value |
|---|---|---|---|
|
All Grade ≥3 AEs |
82 |
68 |
0.010 |
|
SAE |
55 |
63 |
0.21 |
|
Hematologic |
55 |
26 |
<0.0001 |
|
Neutropenia |
46 |
18 |
<0.0001 |
|
Anemia |
11 |
2 |
0.010 |
|
Thrombocytopenia |
9 |
3 |
0.089 |
|
Infection |
13 |
8 |
0.29 |
|
Non-COVID infections |
9 |
14 |
0.21 |
|
COVID |
5 |
4 |
1 |
|
Pneumonia |
3 |
7 |
0.060 |
Higher rates of infection were seen in patients with ≥4 IFM frailty score in both the DR (21%) and Rd (27%) groups, relative to patients with a IFM frailty score of 2–3 (9% and 13%, respectively). Manier comments on a trend towards higher rates of infection in patients with increased frailty in both arms but notes that there were no statistical significance in either.
Conclusion
The IFM 2017-03 trial is a novel phase III clinical trial exploring the possibility of avoiding steroid use for the treatment of frail patients with NDMM. This interim analysis suggests superior rates of ≥VGPR and MRD negativity in patients treated with DR compare with Rd. Safety data for both treatments is comparable to date, with no increased risk of infection or pneumonia with DR despite the significant increase of neutropenia. Further follow-up is ongoing to determine if frail patients with NDMM can be safely treated without steroid-containing regimens.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


