All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
How to treat elderly patients with multiple myeloma in 2020?
During the 6th World Congress on Controversies in Multiple Myeloma (COMy), Alessandra Larocca and María-Victoria Mateos presented on the topic of management of multiple myeloma (MM) in the elderly patients. This article provides a summary of these talks, covering the frailty assessment and the use of proteasome inhibitors (PIs)-based therapy for older patients with MM. Additionally, this article summarizes the session entitled: “Hot debates – Continuous therapy in elderly myeloma?”, by Nizar Bahlis and Suzanne Lentzsch.
Frailty assessment1
MM is a disease of elderly; the incidence rate increases according to age. The latest US Surveillance, Epidemiology and End Results (SEER) registry data reported that 55% of patients are ≥ 75 years of age (1975─2010). The introduction of novel agents has improved the overall survival (OS) in patients with MM, particularly in patients over 65 years with an improvement of the 6-year OS from 31% in 2001─2005 to 56% in 2006─2010 (p < 0.001). However, the outcome of patients ˃ 75 years is still inferior in comparison with the outcome of patients < 75 years.
The elderly population is heterogeneous, but it is possible to distinguish three subgroups of patients, as follows:
- Fit (active and independent)
- Intermediate or unfit (can perform limited activities but are independent)
- Frail (dependent on other people)
In older patients, life expectancy is variable even within the same age group with the number and severity of comorbid conditions and functional impairments being strong predictors of it. Moreover, even the occurrence of severe adverse events affects survival, in particular in frail patients showing higher rates of discontinuation in comparison with fit patients; this may translate in a poorer OS. However, the use of less toxic drug combinations has led to a significantly lower rate of discontinuations (ALCYONE trial, and MAIA trial).
Thus, the complexity of caring for older MM adults arises in part from the heterogeneity of aging, with factors influencing outcomes that include environmental factors (access to care and social support) and patient factors (comorbidities, functional status, and goals of care). To choose the better treatment strategies (choice and duration of treatment, dose and schedule) for unfit and frail patients with MM it is important to detect frail patients.
The International Myeloma Working Group (IMWG) frailty score is the gold standard method for frailty assessment in MM (read more about the classification of frailty here). In frail patients the disease control is possible and beneficial.
PIs-based therapy for the management of MM in the elderly2
For more than 40 years, melphalan plus prednisone (MP) was the standard of care for patients with MM. However, in the last 20 years the proteasome inhibition has been one of the most relevant steps to improve the survival of elderly patients with newly diagnosed MM (NDMM).
Bortezomib (V) is a reversible PI that showed significant benefit, in terms of time to disease progression and OS, in combination with MP in elderly patients with MM in the VISTA trial. Thus, the VMP combination is one standard of care for the management of elderly patients. Based on the VISTA study, the V administration was optimized for the elderly population in two ways: a weekly administration instead of twice-weekly (similar efficacy but less toxicity), and a subcutaneous administration.
- V combinations are also effective in patients with high-risk CA, as observed in the GIMEMA trial where patients treated with the VMP combination showed a longer progression-free survival (PFS)/OS in comparison with those treated with lenalidomide/dexamethasone (Rd)
- The VMP combination has been recently optimized with the addition of the monoclonal antibody daratumumab (D) and this combination has been evaluated in the ALCYONE trial in elderly patients with NDMM. The results of this trial, after a median follow up of 40.1 months, demonstrated significant benefits in terms of PFS, OS and overall response rate in patients treated with daratumumab plus VMP (D-VMP) vs those treated with VMP; and led to the U.S. Food and Drug Administration and European Medicines Agency approval of the quadruplet combination in the treatment of elderly patients with NDMM
- V can also be combined with IMiDs® and the VRd combination has been approved by both U.S. Food and Drug Administration and European Medicines Agency for the treatment of elderly patients with NDMM; the approval was based on the results of the SWOG-S0777 study showing a superiority of the VRd vs Rd treatment in elderly patients
- Ongoing trials are evaluating a further optimization of the VRd combination with the addition of the anti-CD38 antibody D or isatuximab
Carfilzomib (K) is a second-generation PI, administered intravenously. The combination based on K plus MP showed the same PFS and OS as the VMP combination. A phase II randomized trial is evaluating the combinations KRd and K plus thalidomide and dexamethasone (KTd), in non-transplant eligible patients with NDMM, and preliminary results of combined data (KRd + KTd) are promising. Preliminary data showed that almost all patients responded to these combinations (overall response rate = 95.6%) with 30.4% of patients achieving complete response
- KRd and daratumumab plus KRd (D-KRd) are being evaluated in comparison with VMP in fit elderly patients with NDMM
Ixazomib, an oral PI, is being evaluated in combination with D and low dose d in unfit (median age: 76 years) or frail (median age: 82 years) patients in the phase II HOVON 143 study. Preliminary results, with a low early mortality rate (unfit, 2%; frail, 9%), are promising
PIs have been always utilized as part of combinations of fixed duration. But still, is there a role for PIs as maintenance?
- V has been evaluated as maintenance in elderly patients with NDMM in the PETHEMA/GEM05 study. In this study, VMP induction was followed by maintenance with VT or VP. Maintenance with VT/VP increased the complete response rate after VMP up to 42% and both median PFS and OS improved in comparison with the classical VMP of fixed duration. Another study, the GIMEMA MM-03-05 study, showed significant benefit (in both PFS and OS) with VT maintenance after VMPT in comparison with VMP followed by no maintenance
- The Tourmaline-MM4 trial is evaluating ixazomib vs placebo as maintenance in transplant ineligible patients with NDMM, and new data is going to be presented at the 25th European Hematology Association (EHA) Annual Congress
In conclusion, PIs alone or with corticosteroids would be indicated for frail patients. PIs take part of the different multidrug combinations and are good partners for either IMiDs, monoclonal antibodies, or alkylators.
Debate: Continuous therapy in elderly myeloma?3
Continuous therapy commonly refers to administering a regimen until disease progression. The regimen is typically a doublet or triplet, such as standard of care Rd. The MM Hub conducted a poll on Twitter entitled “Is continuous therapy appropriate in elderly patients with MM?”, and the results are shown in Figure 1.
Figure 1. Results of the MM Hub poll on the use of continuous therapy in elderly patients with MM
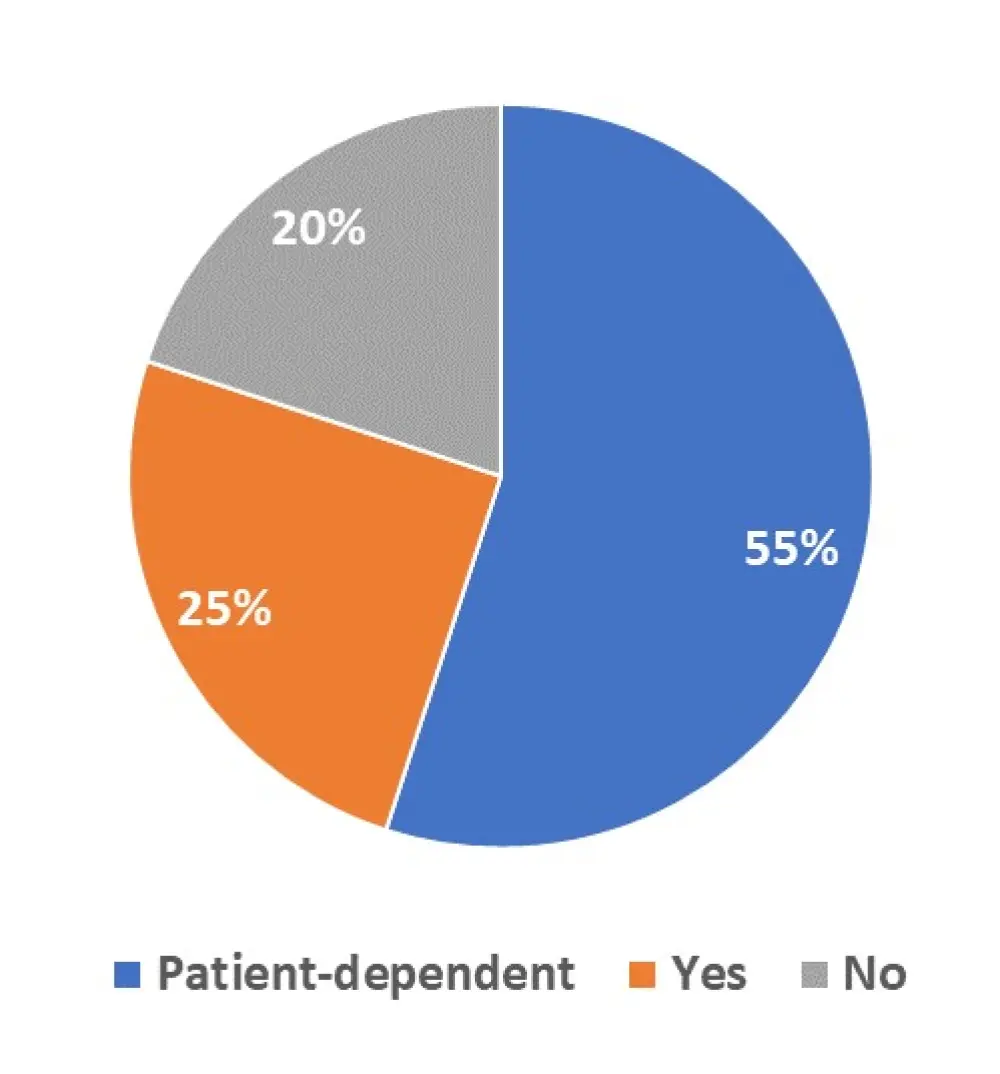
During COMy, Nizar Bahlis and Suzanne Lentzsch debated about the use of continuous therapy in elderly patients with MM. The main arguments in favour or against continuous therapy in elderly myeloma are summarized in Table 1.
Table 1. Summary of debate between Nizar Bahlis and Suzanne Lentzsch
|
CR, complete response; DoR, depth and duration of response; D-Rd, daratumumab plus Rd; D-VMP, daratumumab plus VMP; MP, melphalan/prednisone; MPR, MP and lenalidomide; MPT, MP and thalidomide; OS, overall survival; PFS, progression-free survival; Rd, lenalidomide/dexamethasone; VGPR, very good partial response; VMP, bortezomib, melphalan, and prednisolone; VMPT, VMP and thalidomide; VMPT-VT, VMPT followed by VT |
|
|
Continuous therapy in elderly myeloma? |
|
|
Nizar Bahlis: YES |
Suzanne Lentzsch: NO |
|
Goal: To maximize the rate and durability of measurable residual disease negative state without increasing toxicity, resulting in improved survival outcomes |
It is important to consider the expectations and the life expectancy of the patients and their understanding of the disease; as well as comorbidities, tolerability, and individual circumstances (support system, financial situation, personal lifestyle) |
|
DoR improve with continuous therapy: -The FIRST trial showed an improvement in depth of response with Rd continuous therapy vs Rd 18 months. The DoR, for patients in CR, improved as well with a median DoR of 59.1 vs 40.1 months, and 49 vs 28.1 months for patients achieving VGPR, with Rd continuous therapy vs Rd 18 months, respectively |
The trial design needs to answer the question of length of treatment: -The ALCYONE trial compares nine cycles of VMP vs nine cycles of D-VMP followed by continuous therapy with D. This trial compares VMP vs D-VMP: triplet vs quadruplet, and not continuous vs fixed treatment |
|
Superior PFS outcomes with continuous vs fixed therapy: -FIRST study, comparing Rd continuous therapy vs Rd 18 months vs MPT, median PFS 33.2 vs 23.1 vs 25.8 months in responding patients -GIMEMA MM-03-05 study, comparing VMPT plus VT continuous therapy vs VMP, median PFS 35.3 vs 24.8 months -MM-015 trial, comparing MPR plus R continuous therapy vs MPR vs MP, median PFS 31 vs 14 vs 13 months -MYELOMA XI trial, comparing R continuous therapy vs observation, median PFS 26 vs 11 months in transplant-ineligible patients -MAIA trial, comparing D-Rd plus D continuous therapy vs Rd, median PFS not reached vs 33.8 months -ALCYONE trial, comparing D-VMP plus D continuous therapy vs VMP, median PFS 36.4 vs 19.3 months |
No better patient outcomes with continuous therapy vs fixed therapy: -A phase III study, comparing Rd induction plus R maintenance vs continuous Rd in intermediate-fit elderly patients, showed no difference in PFS and OS between the two arms |
|
Improved quality of life and decreased morbidity by delaying relapse: -The FIRST trial showed that the incidence of adverse events did not increase during months 18─24; the quality of life does not deteriorate beyond 18 months of treatment with Rd |
Increased toxicity in older patients with continuous vs fixed therapy: -FIRST trial, Grade 3/4 infection rate was 32% vs 22% vs 17% in continuous Rd vs Rd 18 months vs MPT -MAIA trial, more patients in the D-Rd group experienced neutropenia (50% vs 35.3% with D-Rd vs Rd, respectively) and Grade 3/4 infections (32.1% vs 23.3% with D-Rd vs Rd, respectively) |
|
Decrease of disease burden over time |
Treatment burden due to repeated trips to hospital, physician appointments, or for repeated intravenous or subcutaneous drug administration and pharmacoeconomic burden |
|
With fixed-duration therapy you can stop treatment at a certain time-point, wait for relapse, then start therapy again, but assuming that: -all patients at relapse will be fit to receive salvage therapy, there are no new comorbidities or disease related complications -the disease biology remains static, no account for clonal dynamics -the patient quality of life is better with fixed duration therapy, but as observed in the FIRST trial this is not true |
|
|
Improvement in OS: -GIMEMA MM-03-05, comparing VMPT-VT vs VMP, 5-year OS 61% vs 51% (p = 0.01) -ALCYONE trial, comparing D-VMP vs VMP, 3-year OS 78% vs 67.9% (p = 0.0003) |
No improvement in OS: -FIRST study, 4-year OS was almost the same in patients who received Rd continuous therapy vs patients who received Rd for a fixed period of time (18 months), 59% vs 58%, respectively |
|
Continuous therapy does not result in the emergence of more aggressive disease at relapse leading to worse survival outcomes -In the MYELOMA XI trial, the percentage of patients gaining a mutation was slightly higher in the observation group than in the R group, and the number of mutation clusters at presentation and relapse was similar in the R group vs the observation group. The PFS until second relapse was still better in the R group vs the observation group (43 vs 35 in the transplant-ineligible population) |
|
Further Resources
The full congress sessions are currently available via this link [Correct as of June 10, 2020].
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

