All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Elderly-fit NDMM: Latest updates from ASH 2023
In multiple myeloma (MM) patients fitness, particularly in elderly populations, is a vital measure to determine an appropriate and safe treatment plan. The International Myeloma Working Group Frailty Score is a geriatric clinical scoring system that has been adapted for use in patients with MM. This score categorizes patients into fit, intermediate-fit, and frail to guide the treatment pathway.1
During the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, Bruno and Mateos presented the latest data from the EMN20 (NCT04096066) and GEM2017FIT (NCT03742297) clinical trials on the treatment of elderly-fit patients, respectively. The Multiple Myeloma Hub is pleased to summarize these presentations below.
EMN201
EMN20 is a phase III, randomized clinical trial investigating carfilzomib-lenalidomide-dexamethasone (KRd) vs lenalidomide-dexamethasone (Rd) in newly diagnosed fit and intermediate-fit, transplant ineligible patients.
Design
The treatment goal for elderly fit and intermediate fit patients is to achieve deep and prolonged responses, which are limited in standard of care Rd therapy. EMN20 aimed to compare the long-term outcomes of treatment with either intervention for this goal.
The trial design of EMN20 is outlined in Figure 1.
Figure 1. EMN20 trial design*
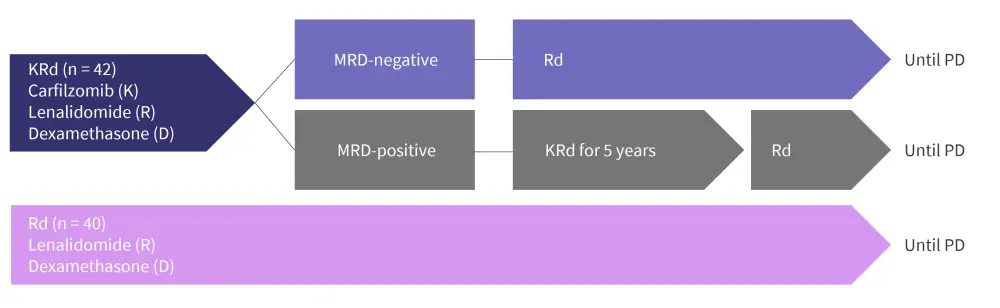
KRd, carfilzomib-lenalidomide-dexamethasone; MRD, measurable residual disease; PD, progressive disease; Rd, lenalidomide-dexamethasone.
*Adapted from Bruno.1
- Primary endpoints include measurable residual disease (MRD) after 2 years of treatment and progression-free survival (PFS)
- MRD assessment: sensitivity of × 10−5
- MRD assessment after 1 and 2 years of study therapy in patients who achieved ≥ very good partial response
- Secondary endpoints include response rates, overall survival, and safety
Results
- MRD negativity rates were observed only in the KRd cohort, with 40% having sustained MRD negativity
- MRD negativity at ≥10−5 sensitivity was not recorded in any patients throughout the follow-up period
Figure 2 outlines the MRD negativity rates observed at 1-year, 2-year, and sustained for both the Rd and KRd cohorts.
Figure 2. MRD negativity ≥10−5 sensitivity over time*
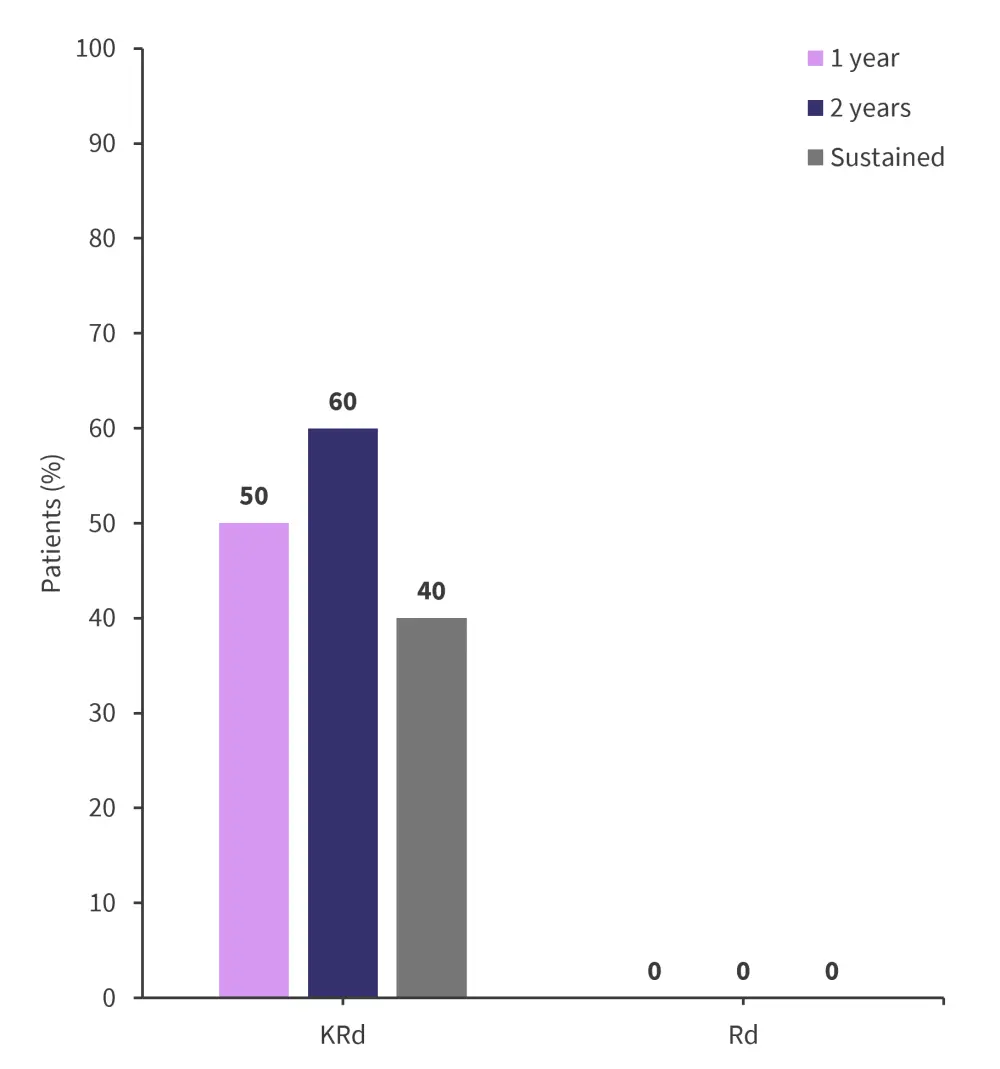
KRd, carfilzomib-lenalidomide-dexamethasone; MRD, measurable residual disease; Rd, lenalidomide-dexamethasone.
*Data from Bruno.1
- Overall response rates, were higher in the KRd cohort vs Rd cohorts, respectively (95% vs 78% as shown in Figure 3)
- There were also significantly higher rates of complete response (CR) and stringent CR observed in the KRd cohort
- PFS rate at 2 years was 81% vs 48% in the KRd and Rd cohorts, respectively
- Median PFS was not reached in the KRd cohort vs 20.9 months in the Rd group
Figure 3. Response rates*
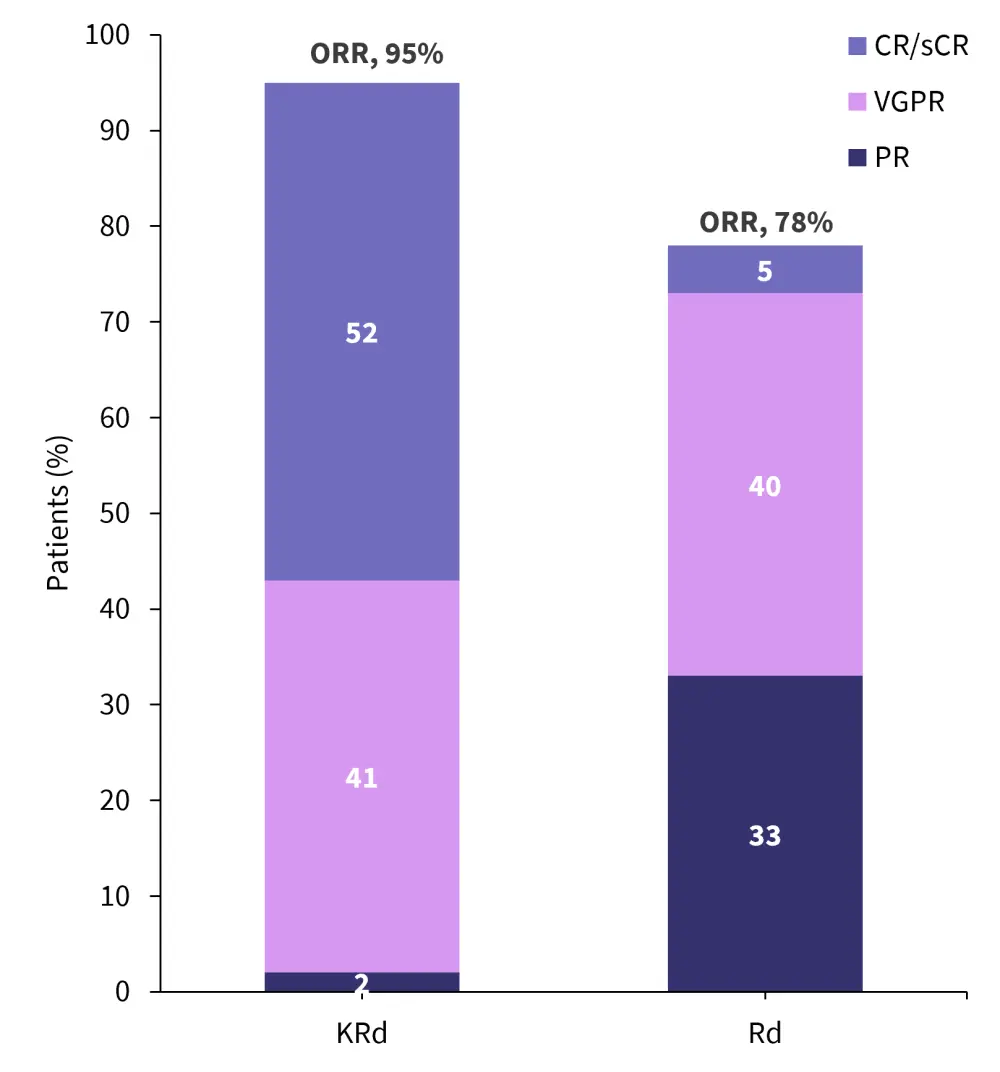
CR, complete response; KRd, carfilzomib-lenalidomide-dexamethasone; PR, partial response; Rd, lenalidomide-dexamethasone; sCR, stringent CR; VGPR, very good PR.
*Adapted from Bruno.1
- An increased number of Grade 3–5 adverse events (AEs) were observed in the KRd cohort, with the most common AEs being hematologic followed by infection.
Table 1. Grade 3–5 adverse events*
|
AE, adverse event; KRd, carfilzomib-lenalidomide-dexamethasone; Rd, lenalidomide-dexamethasone; VTE, venous thromboembolism. |
||
|
Adverse event, % |
KRd |
Rd |
|---|---|---|
|
Anemia |
7 |
0 |
|
Neutropenia |
22 |
12 |
|
Thrombocytopenia |
10 |
2 |
|
Cardiovascular |
12 |
3 |
|
Infections |
17 |
11 |
|
Peripheral neuropathy |
5 |
0 |
|
VTE |
0 |
5 |
|
Skin AEs |
5 |
10 |
|
SARS-COVID19 |
12 |
8 |
Presenter conclusions
The administration of KRd weekly led to higher rates of MRD negativity compared with the Rd regimen, with 40% experiencing sustained negativity. This MRD negativity was also observed to be associated with prolonged periods of PFS in all high-risk groups. The rate of AEs were; however, slightly increased; highlighting the need for observation and management throughout.
GEM2017FIT2
GEM2017FIT is a phase III, randomized clinical trial investigating induction with bortezomib-melphalan and prednisone (VMP) followed by Rd vs KRd with or without daratumumab (D), followed by consolidation and maintenance therapy with lenalidomide and daratumumab in elderly fit.
Design
In total, 462 patients were enrolled and assigned to either:
- VMP-Rd; nine cycles of VMP followed by nine cycles of Rd induction, then four cycles of DRd consolidation (n = 154)
- KRd; 18 cycles of KRd induction followed by four cycles of DRd consolidation (n = 154)
- D-KRd; 18 cycles of induction (n = 153)
The primary endpoint was MRD negativity by next-generation flow at a sensitivity at 10−5 after 18 cycles of induction.
Results
- Both at × 10−5 and × 10−6 sensitivity, the highest rates of MRD negativity were observed in the D-KRd cohort followed by the KRd cohort with both cohorts being significantly improved compared with the VMP-Rd comparator (Figure 4)
- 30 months PFS rate:
- KRd, 83%
- D-KRd, 79%
- VMP-Rd, 73%
Figure 4. MRD negativity rates after 18 induction cycles*
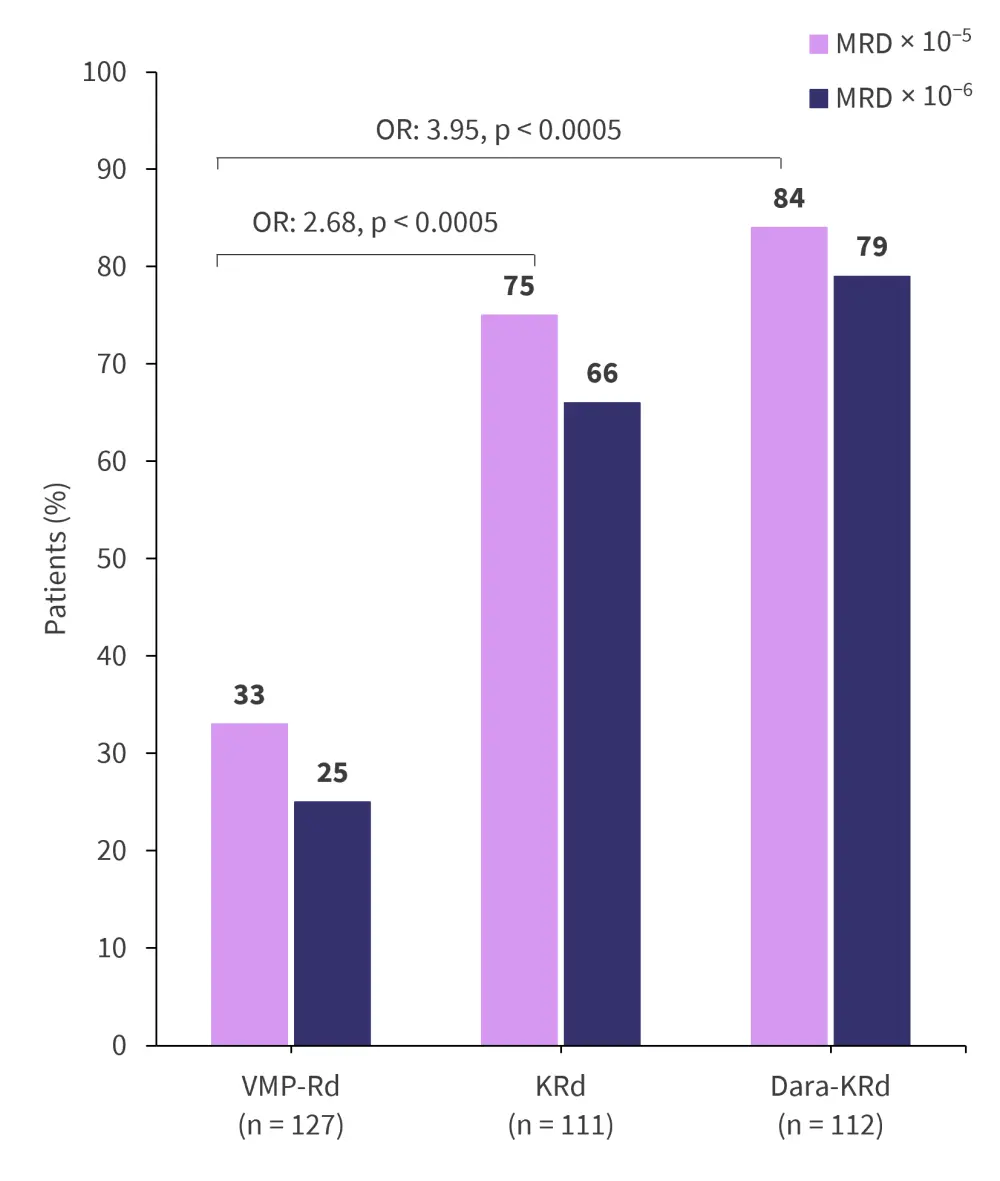
D-KRd, carfilzomib-lenalidomide-dexamethasone-daratumumab; KRd, carfilzomib-lenalidomide-dexamethasone; MRD, measurable residual disease; OR, odds ratio; VMP-Rd, bortezomib-melphalan-prednisone.
*Adapted from Mateos.2
- Overall response rates between the groups were not significantly different; however, the highest overall response rate was observed in the D-Krd followed by KRd groups
- An increase in the number of CR and stringent CR was also higher in the D-KRd paired with a lower proportion of patients only experiencing PR (Figure 5)
Figure 5. Best response*
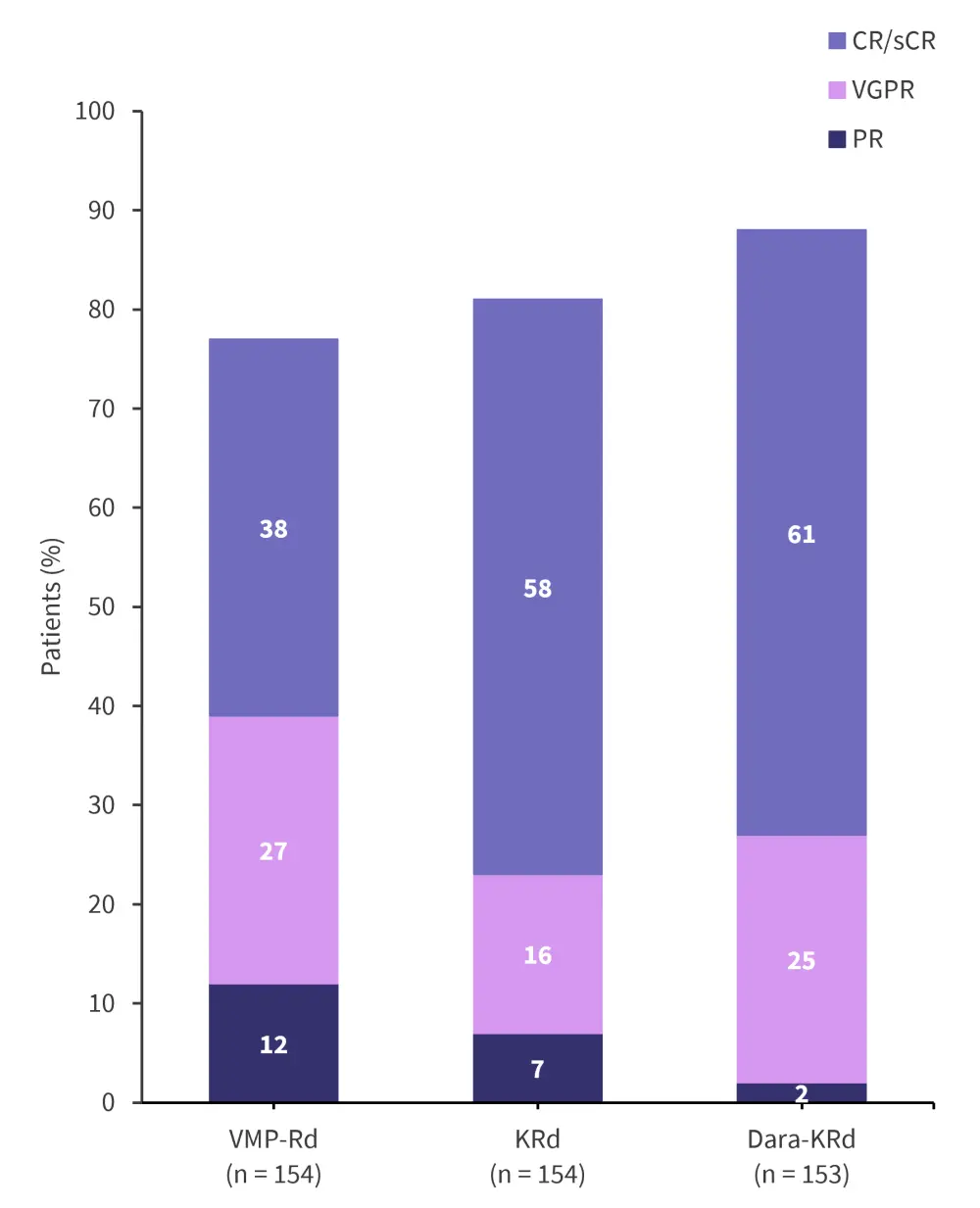
CR, complete response; D-KRd, carfilzomib-lenalidomide-dexamethasone-daratumumab; KRd, carfilzomib-lenalidomide-dexamethasone; ORR, overall response rate; PR, partial response; sCR, stringent CR; VGPR, very good PR; VMP-Rd, bortezomib-melphalan-prednisone.
*Adapted from Mateos.2
- Overall, toxicity profiles were comparable with an increased number of hematologic toxicities associated with VMP-Rd, with neutropenia being the most common Grade 3–4 AE in all three cohorts
Table 2. Grade 3–4 adverse events*
|
D-KRd, carfilzomib-lenalidomide-dexamethasone-daratumumab; KRd, carfilzomib-lenalidomide-dexamethasone; VMP-Rd, bortezomib-melphalan-prednisone. |
|||
|
Adverse event, % |
VMP-Rd |
KRd |
D-KRd |
|---|---|---|---|
|
Hematologic |
|
|
|
|
Neutropenia |
50 |
24 |
47 |
|
Anemia |
11 |
5 |
10 |
|
Thrombocytopenia |
34 |
16 |
17 |
|
Non-hematologic |
|
|
|
|
Gastrointestinal |
9 |
7 |
12 |
|
Infections |
12 |
15 |
16 |
|
Rash |
2 |
12 |
6 |
|
Cardiovascular |
5 |
11 |
14 |
|
Cardiac failure |
2 |
2 |
5 |
|
Hypertension |
— |
5 |
2 |
Presenter conclusions
Treatment with KRd or D-KRd resulted in significantly increased rates of MRD negativity after 18 cycles vs VMP-Rd. These results were mirrored in increased overall response and PFS rates, which were slightly higher than the VMP-Rd group. Toxicities were more common in the KRd arm with progressive disease more common in the VMP-Rd arm, and death-related toxicity in the D-KRd arm.
Overall conclusion
Overall, the treatment of newly diagnosed MM in elderly fit patients is tending towards more triplet and quadruplet therapies with the developing understanding of the myeloma landscape and increasing availability of novel therapies.
The use of KRd has been observed to result in high rates of MRD negativity in both the whole cohort and transplant ineligible groups, with the addition of daratumumab showing evidence of further improvement in the 65–80-year age category. However, longer periods of observation with these combination therapies are required to determine where dose reductions may be appropriate in patients reaching MRD negativity to reduce toxicity rates and improve quality of life.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

