All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
EHA-ESMO clinical practice guidelines for the diagnosis, treatment, and follow-up of MM
The European Hematology Association (EHA) and European Society for Medical Oncology (ESMO) guidelines committees have jointly issued consensus-based recommendations for the diagnosis, treatment, and follow-up of patients with multiple myeloma (MM). These were published in the February 2021 edition of HemaSphere.1
Below, we summarize the key recommendations provided for newly diagnosed and relapsed/refractory (R/R) MM, alongside smoldering MM, plasma cell leukemia, and solitary plasmacytoma.
Diagnosis and staging
The ESMO clinical practice guidelines for the diagnosis, staging, and definitions of myeloma disease, published in 2017, are still applicable.2 Tests required for diagnosis are shown in Figure 1.
Figure 1. Examinations recommended to be performed at diagnosis of MM1
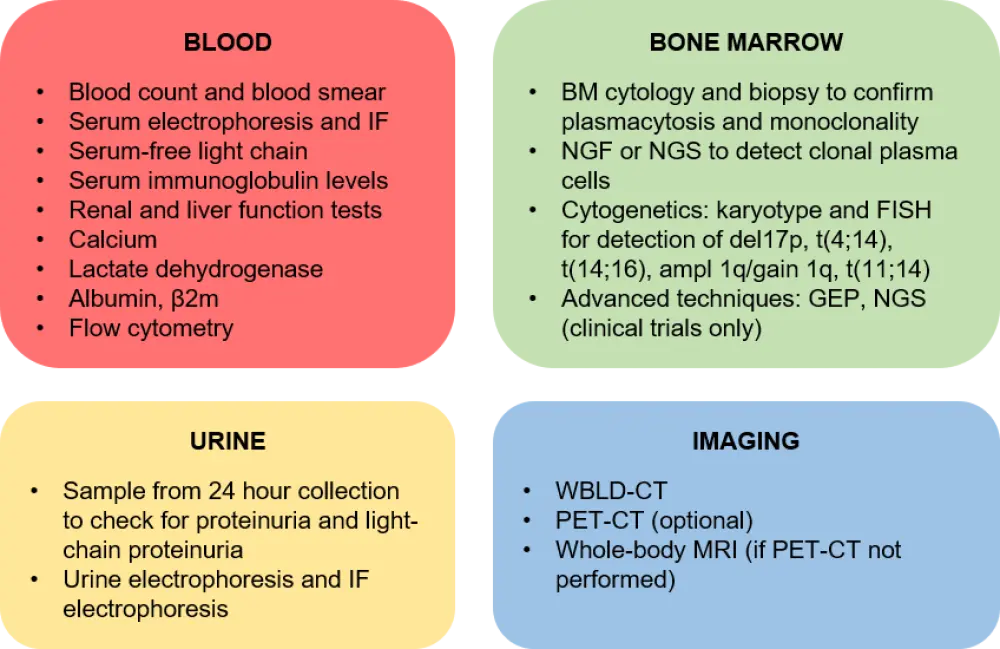
Β2m, beta-2 microglobulin; BM, bone marrow; FISH, fluorescence in situ hybridization; GEP, gene expression profiling; IF, immunofixation; MRI, magnetic resonance imaging; NGF, next-generation flow cytometry; NGS, next-generation sequencing; PET-CT, positron emission tomography-computed tomography; WBLD-CT, whole-body low-dose-CT.
Response criteria to anti-myeloma therapy
- Review the International Myeloma Working Group (IMWG) response criteria here
- Positron emission tomography-computed tomography (PET-CT) is currently considered the best method for imaging minimal residual disease (MRD)
- MRD negativity, defined as the absence of tumor plasma cells within 1,000,000 (< 10−6) or 100,000 (< 10−5) bone marrow (BM) cells, is a surrogate endpoint for progression-free survival in patients receiving first-line treatment. With the international consensus for MRD assessment in MM trials, MRD may guide treatment decisions in the future
Frontline therapy
Smoldering MM
- No treatment is currently approved for patients with smoldering MM, with a watch-and-wait approach recommended for patients with standard- or intermediate-risk smoldering MM
- Participation in randomized clinical trials should be discussed with high-risk patients, defined by the IMWG 2/20/20 risk stratification model for those with two or more of the following risk factors:
- > 2 g/dL serum monoclonal protein
- Serum-free light chain ratio > 20
- BM plasma cells > 20%
- Find more information on the current discussion about treating or not smoldering MM in this previous publication
Patients with NDMM who are eligible for high-dose therapy and auto-HSCT
- The recommended approach for fit newly diagnosed MM (NDMM) patients <70 years with no comorbidities is induction followed by high-dose therapy (HDT) and autologous hematopoietic stem cell transplant (auto-HSCT), then lenalidomide maintenance; as presented in Figure 2
Figure 2. Treatment approach for patients with NDMM who are eligible for auto-HSCT1
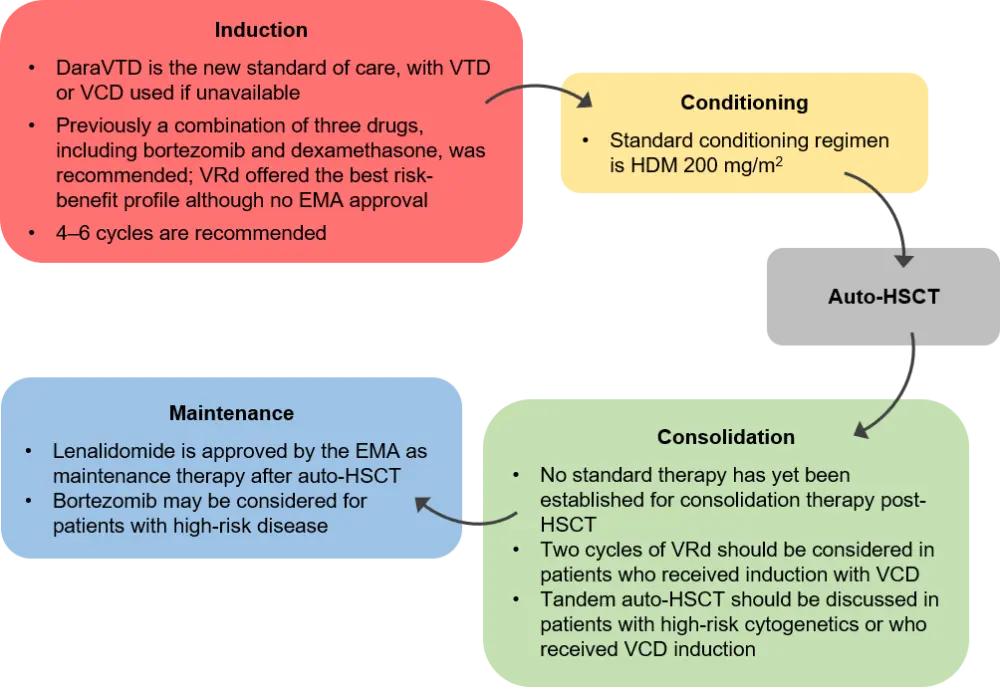
Auto-HSCT, autologous hematopoietic stem cell transplant; DaraVTD, daratumumab plus VTD; EMA, European Medicines Agency; HDM, high-dose melphalan; VCD, bortezomib, cyclophosphamide and dexamethasone; VRd, bortezomib, lenalidomide, and dexamethasone; VTD bortezomib, thalidomide, and dexamethasone.
Elderly patients or patients with NDMM who are ineligible for HDT and auto-HSCT
- The combination therapies daratumumab plus bortezomib, melphalan, and prednisone (daraVMP), daratumumab plus lenalidomide, and dexamethasone (daraRd), and bortezomib, lenalidomide, and dexamethasone (VRd) are all approved by the European Medicines Agency (EMA) in patients ineligible for auto-HSCT
- Rd and VMP may be considered for patients who cannot receive these regimens
- Listen to a comprehensive review on available treatments for elderly patients with NDMM
Treatment of patients with R/R MM
Patients who have received one prior line of therapy (Figure 3)
- There was consensus that a second auto-HSCT should be considered in patients who had initially received auto-HSCT with lenalidomide maintenance and had an initial remission lasting ≥36 months
- Patients who have been given upfront bortezomib-based therapy without lenalidomide or daratumumab should be treated with an Rd-based regimen second-line, for instance, daraRd, KRd, ixazomib (Ixo) plus Rd, or elotuzumab (Elo) plus Rd
- In lenalidomide-refractory patients, pomalidomide (Pom) plus Vd is approved second-line, and daraKd and isatuximab (Isa) plus Kd are also recommended; selinexor (S) plus Vd can also be considered
- Venetoclax plus Vd is an option restricted to patients with t(11;14) who have failed lenalidomide and are sensitive to proteasome inhibitors (PIs)
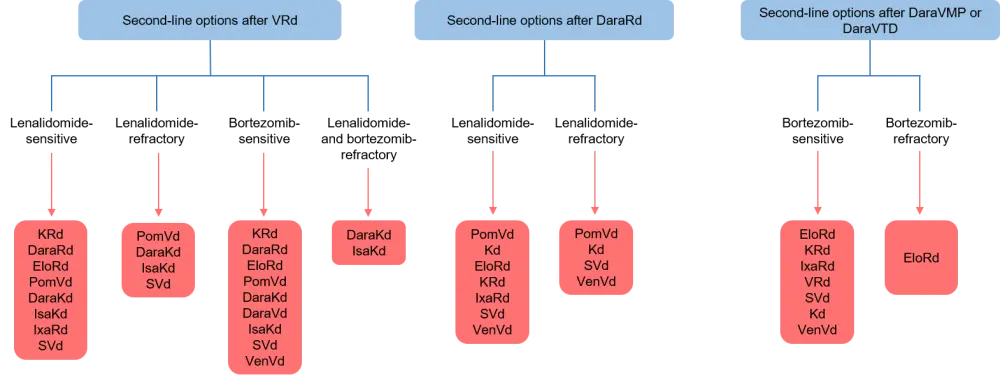
Dara, daratumumab; Elo, elotuzumab; Isa, isatuximab; Ixa, ixazomib; K, carfilzomib; Kd, carfilzomib and dexamethasone; Pom, pomalidomide; Rd, lenalidomide and dexamethasone; S, selinexor; Vd, bortezomib and dexamethasone; Ven, venetoclax; VMP, bortezomib, melphalan, and prednisone; VRd, bortezomib, lenalidomide, and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone.
Patients who have received two or more prior lines of therapy
- Although the management of patients with R/R MM who have received ≥ two lines of prior therapy is challenging, daraKd, isatuximab in combination with pomalidomide and dexamethasone (IsaPd), IsaKd, and EloPd are recommended for those who have been exposed or are refractory to both lenalidomide and bortezomib. As of March 2021, daraPd has also been approved by the U.S. Food and Drug Administration (FDA) but not the EMA
- As above, VenVd is suitable for patients who are refractory to lenalidomide and are PI-sensitive
- For triple-class refractory patients, Sd or belantamab mafodotin monotherapy is recommended
Management of plasma cell leukemia
- Peripheral blood analysis and PET-CT are essential for diagnosis; however, the scarcity of phase III trials makes establishing treatment guidelines difficult
- Immediate treatment initiation is recommended, consisting of induction, tandem auto-HSCT, consolidation, and maintenance therapies
- Bortezomib and/or lenalidomide-based multiphase approaches in combination with chemotherapy, with short treatment-free intervals, should be considered
- In patients <50 years old, myeloablative allogeneic (allo-) HSCT can be discussed if a suitable donor is available; in older patients, reduced-intensity conditioning allo-HSCT, following auto-HSCT, may be appropriate
- Continuous treatment should be administered to patients ineligible for transplant
- In patients with R/R disease, the expert consensus supported switching to drugs not used at frontline setting, favoring combinations with lenalidomide or pomalidomide plus dexamethasone, with carfilzomib, daratumumab, or elotuzumab
- For more information on plasma cell leukemia, listen to this podcast
Management of solitary plasmacytoma
- Sensitive techniques should be used to detect any clonal plasma cells in the BM, the presence of which indicates a high risk of early progression to myeloma and warrants system treatment
- In patients with no clonal plasma cells in the BM, radiotherapy is preferred
Supportive care
- Myeloma complications include bone disease, anemia, BM failure, infections, and renal impairment; recommendations for their management are listed in Table 1.
Table 1. Recommendations for the management of complications associated with MM (adapted from Dimopoulos et al.1)
| PI, proteasome inhibitor. | |
| Complication | Recommendation for management |
|---|---|
|
Bone disease |
|
|
Anemia and BM failure |
|
| Infections |
|
| Renal impairment |
|
Follow-up and long-term implications
- Monthly analysis of full blood count, serum, and urine electrophoresis, serum-free light chain, creatinine, and calcium is recommended
- In relapsed patients with no del17p or gain 1q positivity at diagnosis, fluorescence in situ hybridization analysis for these markers should be performed to detect high-risk MM relapse
- New bone lesions can be detected by whole-body low-dose CT, magnetic resonance imaging (MRI) or PET-CT if bone pain is a concern
Personalized medicine
- Currently, no prognostic factor or staging system is routinely used to tailor therapy to individual patients, except for the presence of t(11;14) in R/R MM patients when venetoclax-based regimens are under consideration.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

