All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
International consensus for MRD assessment in MM clinical trials
Measurable (minimal) residual disease (MRD) has become a widely used readout in multiple myeloma (MM) clinical trials. In addition, it has become an established clinical endpoint and a determinant of patient treatment approaches, yet methods to assess and report MRD lack uniformity. In a plea to overcome inconsistencies and further improve the patient outlook in the MM setting, a panel of MM investigators came together to harmonize MRD assessment across clinical trials.
The resulting consensus was presented at the fourth annual Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Myeloma Intergroup Workshop on MRD and Immune Profiling, during the 61st American Society of Hematology (ASH) Annual Meeting. The outlines were developed in affiliation with the International Myeloma Working Group (IMWG) consensus criteria for response and MRD assessment in MM and were recently published in Leukemia.1 The authors suggest that these guidelines should be adhered to by personnel involved in conceiving and analyzing MM trials worldwide (Figure 1). The Multiple Myeloma Hub is happy to provide a summary.
Figure 1. Summary of the international harmonization for MRD assessment in clinical trials for patients with MM1
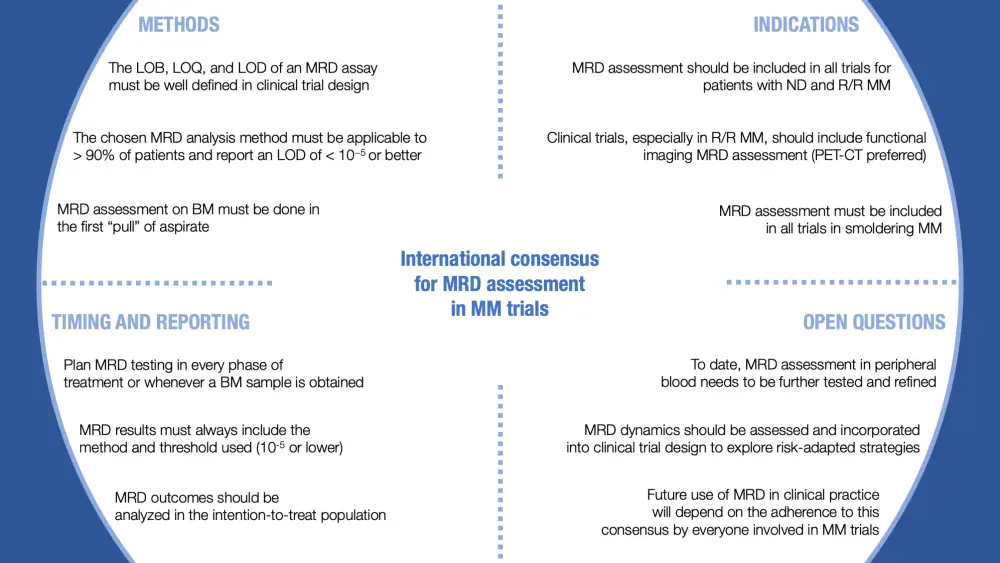
BM, bone marrow; LOB, limit of blank; LOD, limit of detection; LOQ, limit of quantification; MM, multiple myeloma; MRD, measurable residual disease; ND; newly diagnosed; PET-CT, positron emission tomography–computed tomography; R/R, relapsed/refractory.
1. Methodology
Analytical requirements for MRD tests in MM
To ensure the quality of the samples for assessment, analytical validation is required. A suitable validation process should be implemented from collection to MRD readout and should primarily focus on the assay's technical performance. Three parameters are important in defining an assay’s performance:
- limit of blank (LOB; the highest number of aberrant events in a negative sample),
- limit of quantification (LOQ; the lowest number of malignant plasma cells that can be quantified by the assay at a predefined accuracy), and
- limit of detection (LOD; the lowest number of malignant plasma cells that can be detected by an assay consistently).
Key message
The LOB, LOQ, and LOD of an MRD assay must be well defined in clinical trial design, and the reference/analytical assay validation must be included in the clinical trial report.
Performance of MRD assays utilized in MM
Several methods currently employed to assess bone marrow MRD vary in sensitivity and prognostic index. Multiparameter flow cytometry (MFC), next-generation flow cytometry (NGF), and next-generation sequencing (NGS) are particularly sensitive assays with an LOD approaching 10–6, and have been standardized through the EuroFlow consortium and validated by the U.S. Food and Drug Administration (FDA) for MRD assessment.2 However, while NGS requires a baseline sample, which may not be feasible in some patients, MFC does not. On the other hand, samples used in MFC analysis need to be analyzed within 24–48 hours, while samples can be stored for NGS assessment.
Each log of MRD reduction is associated with better outcome, and both NGS and MFC can produce concordant results when using the same threshold. In practice, NGS and MFC have been used successfully for determining MRD status across several clinical trials, including:
Key messages
The chosen MRD analysis method will vary depending on practicality and logistical factors, but those employed in MM clinical trials must be applicable to > 90% of patients and report a LOD of < 10−5. If the assay and sample quality allow, MRD < 10−6 should also be reported.
Bone marrow sampling for MRD assessment
Skilled and experienced professionals should perform sample collection for MRD assessment: hemodilution of the aspirate is seen frequently and could lead to MRD underestimation. The first aspirate (or "pull") should be used for MRD testing. Given that the MRD status has a significant clinical impact, it is important that results are consistent, and the samples collected are of good quality. It is essential to understand the sample requirements for each technique being used, in order to collect the volume of marrow needed and handle samples according to process.
Key messages
MRD assessment on bone marrow must be done in the first “pull” of aspirate. Sample volume and handling must follow the process required by the respective technique for MRD assessment.
2. Indications
Clinical trials in MM with MRD assessment
Several studies in patients with newly diagnosed and relapsed/refractory MM have reported better outcomes for patients who achieved MRD-negative responses. These results provided sufficient evidence to prove that patients in complete response (as defined by the IMWG) can be further stratified by MRD status (at a defined threshold) and duration of their MRD negativity. This led to the inclusion of MRD in the IMWG response assessment in 2016.3
Clinical trials in MM reporting the link between MRD response and clinical outcomes:
Also, novel immunotherapies and cell therapies investigated recently in clinical trials have achieved high rates of MRD negativity in patients with relapsed MM after only a few treatment cycles. Therefore, MRD assessment can also be used in MM trials to evaluate the efficacy of novel agents independently of the classical IMWG response assessment.
Key message
MRD assessment should be included in all trials for patients with newly diagnosed and relapsed/refractory MM.
MRD by imaging
Imaging studies are now crucial complementary techniques for MRD assessments, especially for patients with extramedullary disease or low myeloma plasma cell infiltration in the bone marrow. Positron emission tomography–computed tomography (PET-CT) proved to be a reliable and efficient method for MRD detection and prediction of outcome in the IFM2009 trial (NCT01191060) and in CASSIOPET (a companion study to the CASSIOPEIA phase III trial). However, there is low concordance between PET-CT and MRD assessment from bone marrow. Using a threshold of < 10–5 in the bone marrow for MRD negativity, the CASSIOPET study demonstrated that patients who were both PET-CT and MRD negative experienced longer progression-free survival than those who were PET-CT positive or bone marrow MRD positive. Similar results were also obtained in the IMF2009 trial.
PET-CT is considered to be as sensitive as magnetic resonance imaging (MRI), but it may be clinically more relevant, since it can also detect active disease. However, diffusion-weighted MRI might be a new solution that can detect active residual lesions with even higher sensitivity than PET-CT.
Key messages
Clinical trials, especially in the relapse setting, should continue to incorporate bone marrow and functional imaging MRD assessment simultaneously at predefined time points, due to the additional information that discordant results can provide. PET-CT is the recommended technique since there are already validated data generated by large prospective trials.
Clinical trials in smoldering MM with MRD assessment
Currently, both curative and progression-delay treatment strategies are being tested in smoldering MM to prevent organ damage. MRD monitoring is critical in those trials, as classical endpoints such as progression-free survival may be difficult and cumbersome to assess in these patients. Using MRD as an endpoint to evaluate efficacy for these early interventions may help to indicate potential cure at an earlier time point.
Key message
MRD assessment must be included in all trials in smoldering MM with curative intent.
3. Timing and reporting
Timepoints for MRD assessment in clinical trials
Currently, there is significant heterogeneity when MRD is assessed, which makes comparisons across trials and definitive conclusions on MRD kinetics difficult. For that reason, this panel of MM experts recommends to include MRD testing in every phase of treatment (i.e., induction, transplantation, start of maintenance) or, if the experimental treatment is continuous, include MRD evaluation in every planned bone marrow examination. Alternatively, MRD assessment can be done at predefined time points, such as every 3 months, and can then be gradually delayed to yearly readouts. This might be particularly interesting for therapies administered until disease progression.
Key messages
Plan MRD testing in every treatment phase or whenever a bone marrow sample is obtained. For maintenance or treatment until progression, MRD should be assessed periodically as long as the patient is in complete response.
Proper annotation of MRD results
Reporting MRD results only as "positive" or "negative" has many limitations, although it facilitates the translation of the results into clinical practice, prognosis, and treatment decisions. The most commonly used threshold is 10–5 however, the actual threshold used to analyze MRD results must always be reported, and not assumed from the LOD of the technique used. The panel recommends the following statements when reporting MRD results:
- “X% of patients reached NGS-MRD < 10–5".
- “The rate of NGF-MRD < 10–5 was Y%".
- Although discouraged, the use of terms like "negative" or "positive" should include a statement on the threshold for MRD negativity.
Key messages
When reporting MRD results, the method and threshold used must always be disclosed. Even though this might be improved with the latest techniques, the IMWG and European Medicines Agency consensus uses a threshold of 10–5.
How to report MRD outcomes
The IMWG guidelines for response assessment recommend MRD testing in the subset of patients achieving a complete hematological response. However, previous publications found that some patients might have undetectable MRD with less than a traditional complete response. Investigators usually explain this finding, which is particular to MM, with patchy distribution, extramedullary disease, delayed clearance of the monoclonal protein, or an error in sampling.
Whatever the method employed for MRD assessment, all patients should be included when reporting MRD results (intent-to-treat approach). Those who were not tested will be considered as MRD > 10–5. The panel discourages the use of terms such as “MRD-evaluable population”.
Key messages
Patients who do not undergo MRD assessment must be considered as having MRD > 10–5 (or the applicable threshold for MRD positivity) and must be included in the denominator when reporting MRD-related endpoints.
4. Open questions
MRD in peripheral blood
Several studies investigated the feasibility of assessing MRD in the peripheral blood by the presence of circulating tumor plasma cells, analysis of cell-free DNA, or monoclonal protein detection by mass spectrometry analysis. To date, measurements of circulating tumor plasma cells and cell-free DNA in peripheral blood by MFC and NGS are less sensitive than bone marrow; however, initial results using mass spectrometry are promising, with an LOD close to 10–5. If these techniques, used either alone or in combination, could effectively assess MRD in peripheral blood, this would significantly facilitate residual disease detection in patients with extramedullary disease or patchy distribution.
Key message
MRD assessment in peripheral blood needs to be further tested and refined before it is validated and compared with MRD assessment from bone marrow.
Clinical trials with MRD-guided treatment decisions
The objective of these trials is to employ MRD for the identification of patients at the lower and higher risk of relapse in order to plan a de-escalation or intensification of treatment, thus optimizing the risk–benefit ratio of therapy for each patient. Nevertheless, to date, MRD status is assessed mainly for prognostic purposes, and its use for therapeutic decisions is limited to clinical trials (with several studies ongoing).
Key messages
MRD dynamics should be assessed and incorporated into clinical trial design to guide personalized treatment decisions, such as intensification for patients at higher risk, and de-escalation to spare toxicity for patients at lower risk.
Conclusion
The main goal of this international harmonization is to improve the quality and reproducibility of MRD results in MM-related clinical research. If all the stakeholders involved in clinical trials adhere to these guidelines, MRD can not only be used as a prognostic marker and guide for personalized treatment decisions, but also as a surrogate marker for survival endpoints which would allow earlier approval of novel agents. However, to achieve this, raw data from multiple clinical trials need to be collected and evaluated, a venture that is currently being carried out by the International Independent Team for Endpoint Approval of Myeloma MRD (I2TEAMM).4
Expert Opinion
How significant is the role of MRD status in MM, and how can we best utilize this information?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

