All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Suggested update to current IMWG complete response criteria for MM
Response assessment in multiple myeloma (MM) is guided and classified based on the criteria set out by the International Myeloma Working Group (IMWG) in 2016.1 With the current advances in MM therapies and the increasing number of patients achieving a complete response (CR), a uniform application of response criteria is paramount. According to the IMWG, CR is defined as negative serum and urine M-protein immunofixation (IF), along with the presence of < 5% plasma cells (PCs) in bone marrow (BM) biopsy.1 The same guidelines state that in cases where only the BM fulfils the CR criteria, a second BM biopsy is recommended at the time of serum and urine IF negativity.
Recently, Tschautscher et al. challenged the need for a second BM biopsy to confirm CR in patients who will be undergoing autologous stem cell transplantation (ASCT).2 The results of this retrospective study were published in Blood Cancer Journal and are discussed below, together with a summary of the current IMWG 2016 criteria.
IMWG 2016 response criteria & new suggestion
The current IMWG response criteria (2016) for MM1 and the novel suggestion from Tschautscher et al.2 are depicted in Figures 1 and 2, below.
Figure 1. IMWG (2016) response criteria for MM and new suggestion1,2
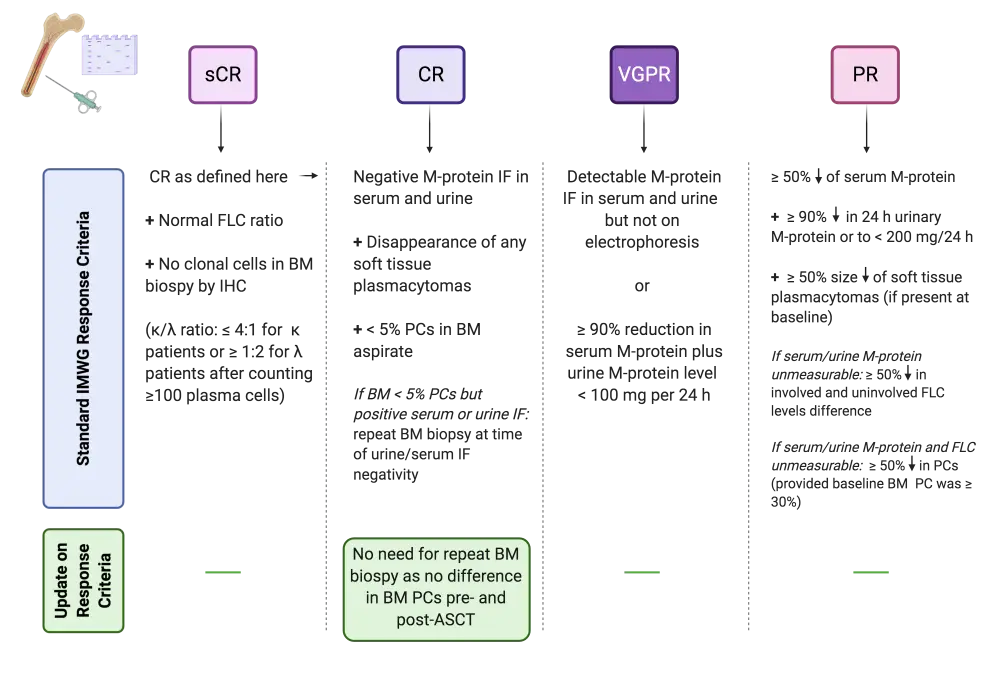
Figure 2. IMWG (2016) response criteria for MM and new suggestion (continued)1,2
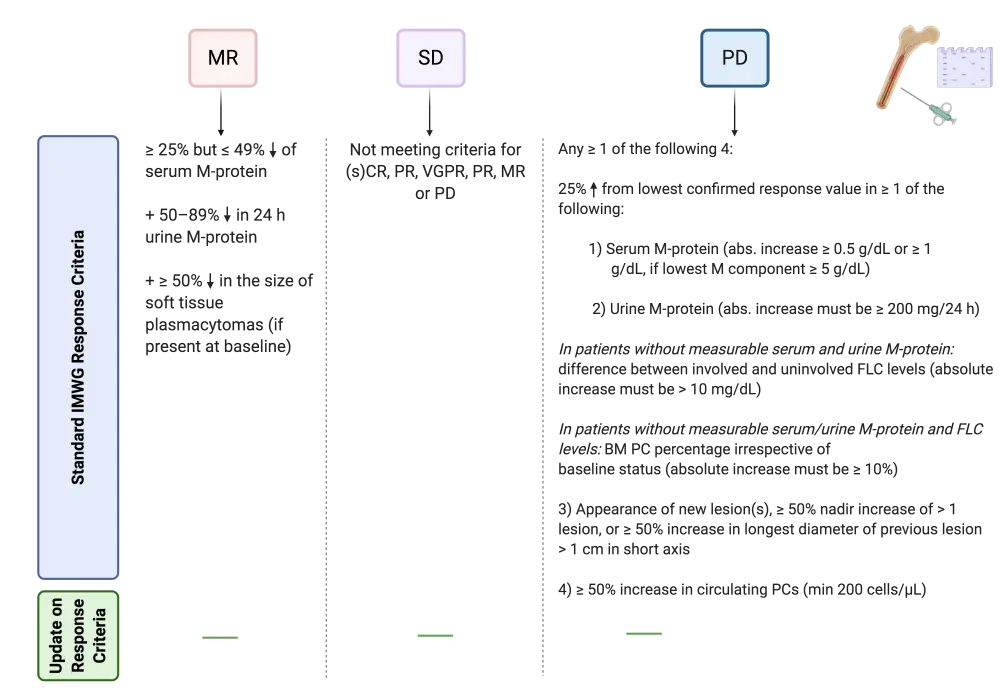
The newly published suggestion for incorporation in the IMWG criteria states that a repeated BM biopsy for CR confirmation is unnecessary. This conclusion was drawn from a retrospective cohort study of 277 patients who underwent ASCT between 1998–2016. All included patients had pre-ASCT IF positivity and < 5% BM PCs, with a median PC level of 0.55% (range, 0.1–29). Following ASCT, 58% of patients remained IF positive in either serum or urine. A second BM biopsy was performed at a median of 3.3 months (range, 1.7–4.9) following ASCT.
In patients with post-ASCT IF negativity (serum and urine; n = 116):
- 98% had no difference in BM PC levels (< 5%)
- Two patients had a BM PC level of 7% and 15%, respectively (both had 6% clonal PCs)
In patients with post-ASCT IF positivity (n = 161):
- 94% had no difference in BM PC levels (< 5%)
- 8.7% had an increase in BM PC levels (median: 7.5%)
- Twelve of them had detectable clonal PCs (median: 5.6%)
Discussion
The results of this prospective study indicate that there might be no need for a repeat BM biopsy after transplantation in order to confirm CR in patients who do not fulfil the IF criteria before transplantation. The authors hope to incorporate this new suggestion into the IMWG response criteria, since it would spare many patients with MM from painful and burdensome BM biopsies.
Limitations of the study include the exclusive evaluation of the CR response criteria and not for stringent CR, and the lack of imaging data on the status of soft tissue plasmacytomas pre- and post-ASCT.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

