All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Do you know... Which of the following drugs results in the highest percentage of cereblon in the closed conformation at saturating concentrations?
Many treatment options are now available for multiple myeloma (MM), with immunomodulatory agents such as lenalidomide and pomalidomide commonly used in combination regimens in both newly diagnosed (ND) and relapsed/refractory (RR) settings.1 Lenalidomide and pomalidomide modulate cereblon within an E3 ubiquitin ligase complex to promote the proteasomal degradation of MM-related proteins.1 However, resistance remains a challenge and necessitates the development of novel agents to improve patient outcomes and treat relapsed disease.1
Novel next-generation cereblon E3 ligase modulatory drugs (CELMoDs) currently under clinical development are designed to improve potency and selectivity toward proteins targeted for degradation by cereblon, with greater antimyeloma and immune-stimulating activity compared with classic immunomodulatory agents.2
Two CELMoDs are in clinical development for MM, iberdomide and mezigdomide. Here, we provide a review of their mechanism of action and an overview of phase III clinical trials currently investigating these agents in RRMM.
Mechanism of action
Advancements in our understanding of the pathogenesis of MM have identified the ubiquitin-proteasome system as a therapeutic target.1 In healthy cells, this system regulates intracellular protein synthesis and degradation; however, in MM cells, this system is dysregulated and promotes cell growth and survival by increasing the degradation of pro-apoptotic and tumor suppressor proteins.1 In the ubiquitin-proteasome system, multiple E3 ligases function as receptors for different intracellular protein substrates, which are then polyubiquitinated and recognized by the 26S proteasome for degradation.1 Cereblon is part of the cullin 4a-cereblon cullin-RING ligase (CRL4CRBN) E3 ubiquitin ligase complex, which is crucial for the ubiquitination of specific target proteins.1 Modulating cereblon has proven to be an effective mechanism for antimyeloma activity.1
Iberdomide and mezigdomide alter the conformation of cereblon within the E3 ligase substrate receptor, switching it from an ‘open’ to ‘closed’ state.1 This causes the CRL4CRBN E3 ligase to recruit neosubstrates for degradation, including two critical lymphoid transcription factors, Ikaros and Aiolos (Figure 1).1,3 Ikaros and Aiolos have an essential role in the development and differentiation of hematopoietic cells and in the survival of MM cells.1 Ikaros and Aiolos also have functions in regulating immune cell activity, including the suppression of the expression of interferon-stimulating genes and the inhibition of T-cell stimulation.1,4,5
Ikaros and Aiolos are recruited when cereblon is in its closed conformation, and the percentage of cereblon in the closed conformation determines the degradation efficiency and antimyeloma activity of CELMoDs.1 Iberdomide and mezigdomide exhibit enhanced cereblon-binding potency over immunomodulatory agents, such as lenalidomide and pomalidomide, due to differences in structural interactions, with increased interactions outside of the thalidomide-binding pocket and their administration as a single S isomer rather than the less efficiently binding mixture of S and R isomers.1 This is evidenced by the higher percentage of cereblon in the closed conformation; at saturating concentrations, 20% of cereblon is in the closed conformation with pomalidomide, compared with 50% and 100% with iberdomide and mezigdomide, respectively.1
The downregulation of Ikaros and Aiolos results in enhanced cytotoxic effects in myeloma cells in vitro, while stimulating T-cell activity, enhancing interleukin-2 and interferon-γ production and increasing natural killer cell proliferation (Figure 2).5,6 The antimyeloma activity of CELMoDs has been observed in cells that are resistant to lenalidomide and pomalidomide.6
Figure 1. Mechanism of action of CELMoDs*
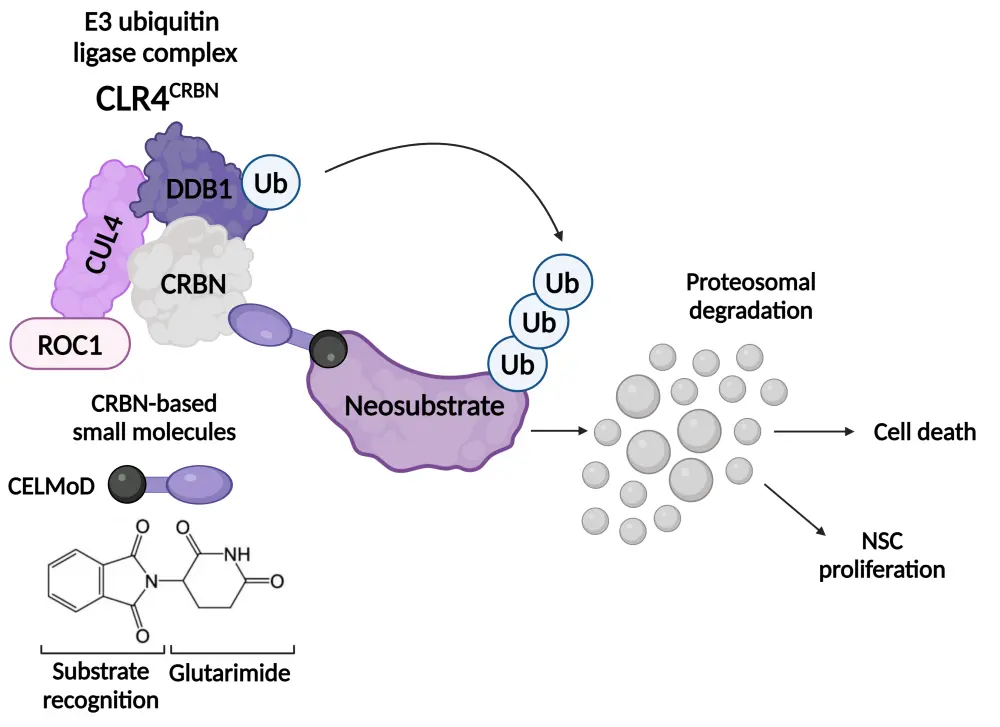
CELMoD, cereblon E3 ligase modulatory drug; CRBN, cereblon; CRL4, cullin 4-RING ubiquitin ligase; CUL4, cullin-RING-based E3 ubiquitin-protein ligase; DDB1, damage-specific DNA-binding protein 1; NSC, neural stem cell; ROC1, regulator of cullins 1; Ub, ubiquitin.
*Adapted from Sato, et al.3 under the Creative Commons Attribution License (CC BY).
Created with BioRender.com
Figure 2. Immunomodulatory effects of IMiDs and CELMoDs in MM*
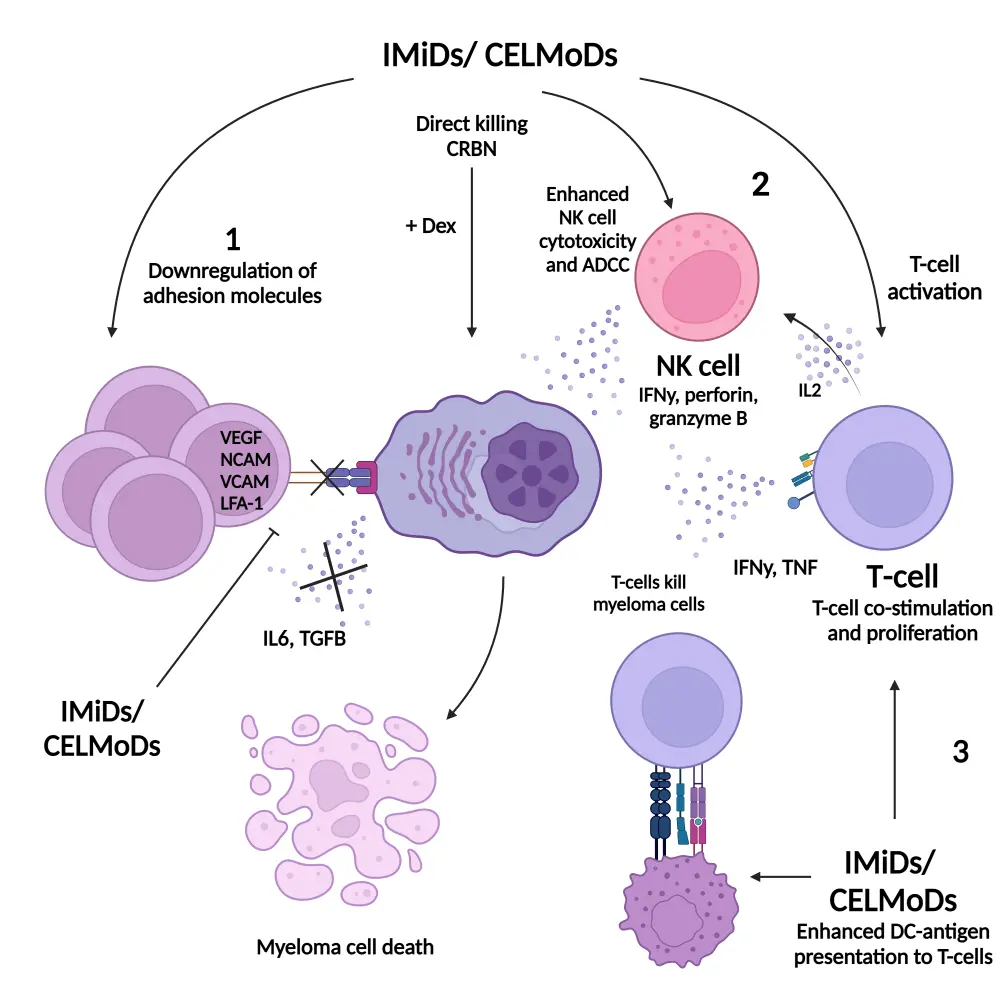
ADCC, antibody-dependent cellular toxicity; BMSC, bone marrow mesenchymal stem cells; CELMoD, cereblon E3 ligase modulatory drug; CRBN, cereblon; DC, dendritic cell; Dex, dexamethasone; IFN, interferon; IL, interleukin; IMiD, immunomodulatory drug; LFA, lymphocyte function-associated antigen; NCAM, neural cell adhesion molecule; NK, natural killer; ROC1, regulator of cullins 1; TCR, T-cell receptor; TGF, transforming growth factor; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
*Adapted from D’Souza, et al.4 under the Creative Commons Attribution License (CC BY).
Created with BioRender.com
Question 1 / 1
Which of the following effects does the downregulation of Ikaros and Aiolos with CELMods result in?
A
Enhanced cytotoxic effects in myeloma cells
B
All of the above
C
Increased natural killer cell proliferation
D
Stimulation of T-cell activity
Efficacy and safety
Iberdomide
The phase I/II CC-220-MM-001 trial (NCT02773030) assessed the safety and efficacy of iberdomide plus dexamethasone in adult patients with RRMM. Phase I was the dose-escalation phase to determine the recommended phase II dose (RP2D) of iberdomide plus dexamethasone, while phase II was the dose-expansion phase.5 This study also included cohorts evaluating iberdomide monotherapy and iberdomide in combination with dexamethasone plus bortezomib (IberVd), carfilzomib (IberKd), or daratumumab (IberDd).5 Patients in the dose-escalation cohort had ≥2 prior lines of therapy, including lenalidomide or pomalidomide and a proteasome inhibitor.5 In the dose-expansion cohort, patients had ≥3 prior lines of therapy and were refractory to lenalidomide or pomalidomide, proteasome inhibitors, CD38 monoclonal antibodies, and corticosteroids.5 In total, 90 and 107 patients were enrolled in the phase I and phase II iberdomide plus dexamethasone cohorts, respectively, while 145 patients were enrolled in the other cohorts.5 The median age was 65 years and 64 years in the iberdomide plus dexamethasone dose-escalation and dose-expansion cohorts, respectively.5
Among patients who received iberdomide plus dexamethasone, in the dose-escalation cohort, patients received oral iberdomide at doses ranging from 0.3 mg to 1.6 mg on Days 1–21 plus oral dexamethasone at 40 mg and 20 mg for patients aged ≤75 years and ≥75 years, respectively, on Days 1, 8, 15, and 22 of each 28-day cycle.5 In the dose-expansion cohort, patients were treated with iberdomide at the RP2D plus dexamethasone at the same dose level as the dose-escalation cohort.5 The median follow-up was 5.8 months and 7.7 months in the dose-escalation and dose-expansion cohorts, respectively.5
In the dose-escalation cohort, 1.6 mg iberdomide was selected as the RP2D in combination with dexamethasone.5 The overall response rate (ORR) was 32% in the dose-escalation cohort (Figure 3).3 In a post hoc analysis of 37 patients in the dose-escalation cohort who received ≥3 prior lines of therapy and were triple-class refractory, the ORR was 30% (Figure 3).5 The median time to response and median duration of response (DoR) were 8.1 weeks and 10.4 months, respectively.5
The ORR in the dose-expansion cohort was 26% (Figure 3).5 The median time to response and median DoR were 4.2 weeks and 7.0 months, respectively.5 The estimated median progression-free survival (PFS) and overall survival (OS) were 3.0 months and 10.7 months, respectively.5 The adverse events (AEs) of interest reported in patients in the iberdomide plus dexamethasone dose-escalation and dose-expansion cohorts are shown in Table 1.
Figure 3. ORR in the iberdomide plus dexamethasone cohorts of the phase I/II CC-220-MM-001 trial*
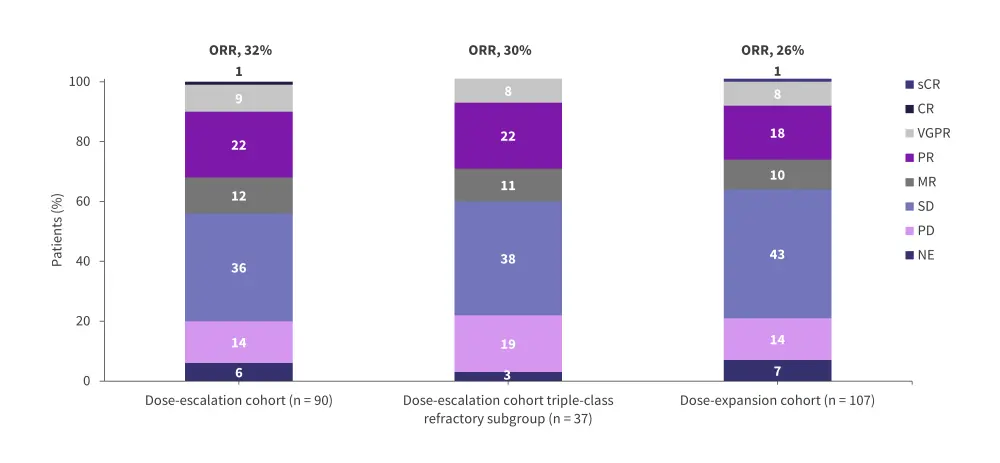
CR, complete response; MR, minimal response; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
*Adapted from Lonial, et al.5
Table 1. AEs reported in the iberdomide plus dexamethasone dose-escalation and dose-expansion cohorts of the phase I/II CC-220-MM-001 trial*
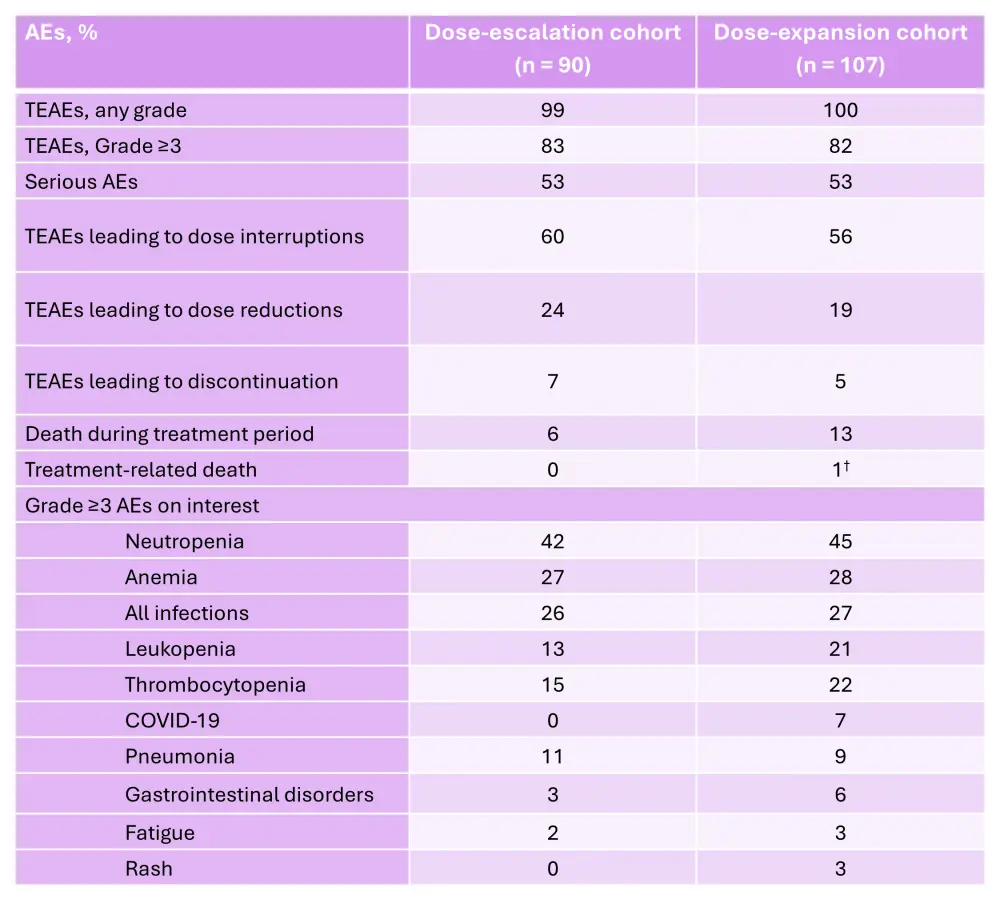
AE, adverse event; TEAE, treatment-emergent adverse event.
*Adapted from Lonial, et al.5
†Due to abdominal sepsis.
Question 1 / 1
What was determined to be the recommended phase II dose of iberdomide when combined with dexamethasone in the dose-escalation part of the phase I/II CC-220-MM-001 trial?
A
1.6 mg
B
1.2 mg
C
0.6 mg
D
0.3 mg
In the IberDd (n = 43), IberVd (n = 25), and IberKd (n = 9) cohorts, the ORRs were 46%, 56%, and 50%, respectively.7 These included a very good partial response (VGPR) or better response in 24%, 28%, and 38% of patients, respectively.7 The median times to response were 4.1 weeks, 3.6 weeks, and 4.1 weeks, respectively.7 The median DoR was 35.7 weeks in the IberVd cohort, and it was not reached in the IberDd and IberKd cohort.7 The hematological Grade 3–4 treatment-emergent AEs of interest included neutropenia (67%), leukopenia (23%), anemia (21%), and febrile neutropenia (5%) in the IberDd cohort, neutropenia (28%) and thrombocytopenia (24%) in the IberVd cohort, and lymphopenia (44%) and neutropenia (33%) in the IberKd cohort.7
The ongoing phase II EMN26 trial (NCT04564703) is assessing the safety and efficacy of iberdomide as a maintenance therapy following autologous hematopoietic stem cell transplantation (auto-HSCT) in patients with NDMM.8 The trial design and key eligibility criteria are shown in Figure 4. Initial results from this trial were reported previously by the MM Hub. Briefly, patients in Cohort 1 and 2 experienced improvements in response after 6 and 12 cycles (Figure 5).8 The PFS rates at 12 months in the 1.3 mg and 1.0 mg cohorts were 91% and 90%, respectively.8 The rates of AEs reported were similar between the 1.3 mg and 1.0 mg cohorts (Table 2).8
Figure 4. EMN26 trial design*
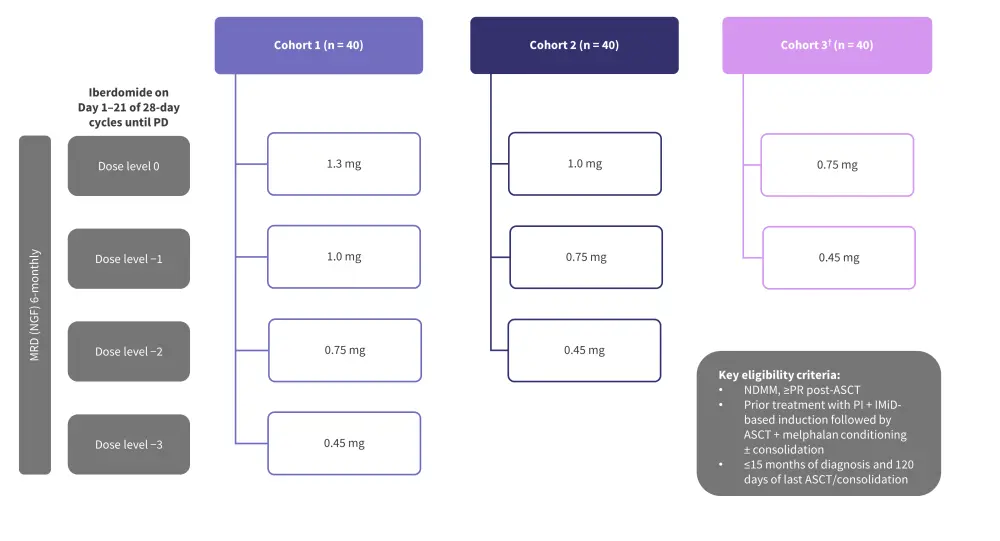
ASCT, autologous stem cell transplantation; IMiD, immunomodulatory agent; MRD, measurable residual disease; NDMM, newly diagnosed multiple myeloma; NGF, next-generation flow; PD, progressive disease; PI, proteasome inhibitor; PR, partial response.
*Adapted from Van de Donk.8
†Cohort 3 was added at a later stage.
Figure 5. Response improvement within A 6 and B 12 treatment cycles in the EMN26 trial*
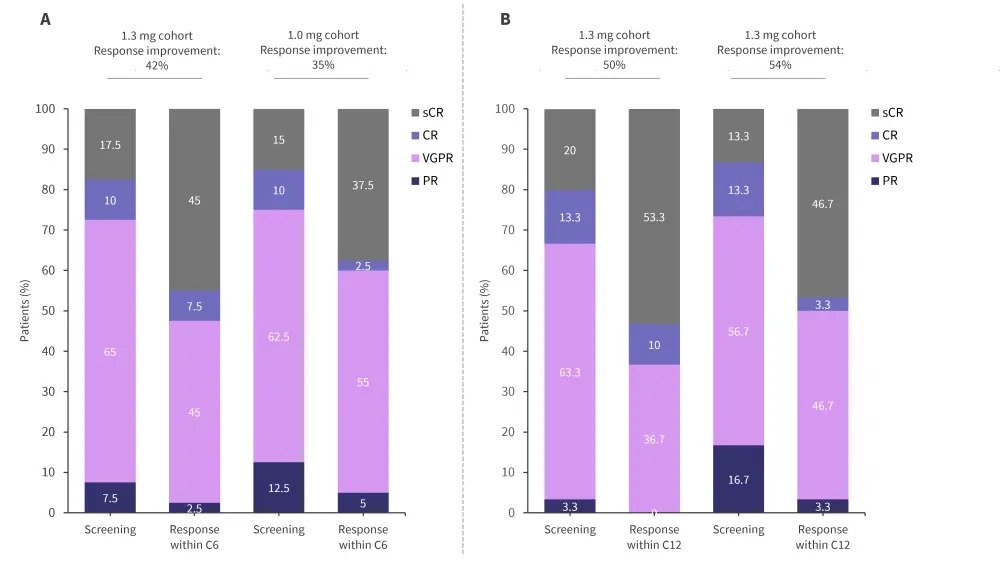
C, cycle; CR, complete response; PR, partial response, sCR, stringent CR; VGPR, very good PR.
*Adapted from Van de Donk.8
Table 2. AEs reported in the EMN26 trial*
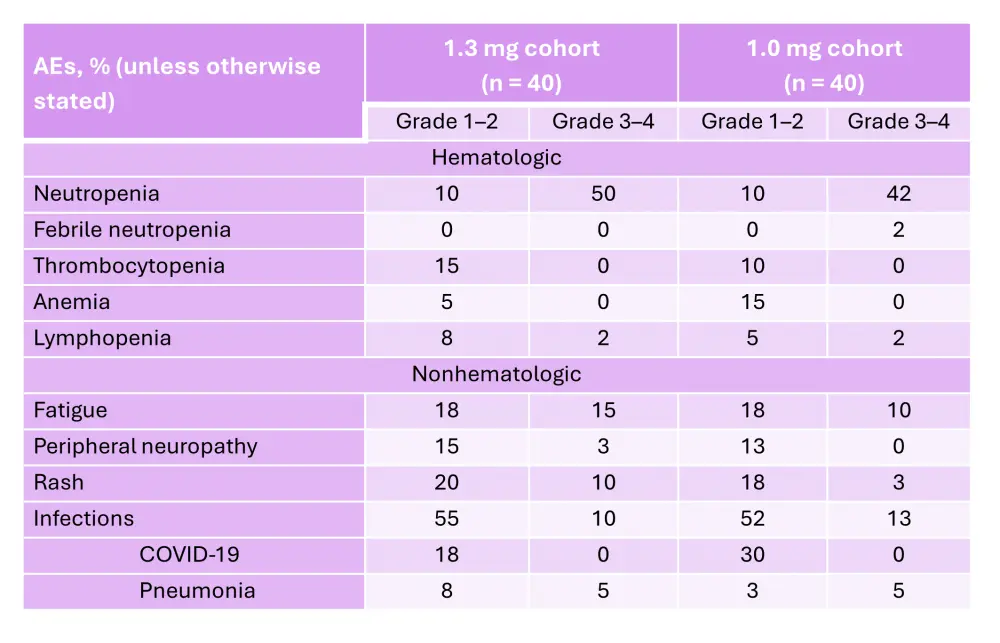
AE, adverse event.
*Adapted from Van de Donk.8
The MM Hub previously spoke with Sagar Lonial, Winship Cancer Institute of Emory University, Atlanta, US. We asked, what are the key efficacy and safety data from pivotal trials of iberdomide for the treatment of MM?
Iberdomide – efficacy and safe
Mezigdomide
The phase I/II CC-92480-MM-001 trial (NCT03374085) evaluated multiple doses and schedules of mezigdomide plus dexamethasone in heavily pretreated patients with RRMM.6 Patients in the phase I (dose-escalation) cohort were aged ≥18 years and had received ≥3 prior lines of therapy, including lenalidomide, pomalidomide, a proteasome inhibitor, a glucocorticoid, and an anti-CD38 antibody.6 Patients in the phase II (dose-expansion) cohort were additionally required to be refractory to these therapies.6
In the dose-escalation cohort, two continuous dosing schedules and two intermittent-intensive schedules were evaluated.6 The continuous dosing schedules included either 10 consecutive days of treatment and 4 days off or 21 consecutive days of treatment and 7 days off, both in 28-day cycles.6 The intermittent-intensive schedules included either 3 consecutive days of treatment followed by 11 days off or 7 consecutive days of treatment and 7 days off, both in 28-day cycles.6 In the dose-escalation cohort, the dose and schedule could be adjusted based on real-time biomarker data.6 In total, 77 and 101 patients were enrolled in the dose-escalation and dose-expansion cohorts, respectively.6 The median age was 65 years and 67 years in the dose-escalation and dose-expansion cohorts, respectively.6
In the dose-escalation cohort, the RP2D was determined to be 1.0 mg of mezigdomide plus dexamethasone once daily in a 21-day schedule.6 In the dose-escalation and dose-expansion cohorts, the ORR was 25% and 41%, respectively (Figure 6 and Figure 7).6 In the dose-expansion cohort, the subgroups of patients with plasmacytomas and previous anti-B-cell maturation antigen (BCMA) therapy showed ORRs of 30% and 50%, respectively (Figure 7).6 The median DoRs were 6.0 months and 7.6 months in the dose-escalation and dose-expansion cohorts, respectively.6 The median DoR in patients who received prior anti-BCMA therapy was 6.9 months. Among patients who had high-risk cytogenetic abnormalities in the dose-expansion cohort, the ORR was 32% and the median DoR was 10.0 months.6 The median PFS in the dose-expansion cohort was 4.4 months, 5.4 months, and 2.8 months, for all patients, patients who received prior anti-BCMA therapy and who had high-risk cytogenetic abnormalities, respectively.6 The AEs reported in the dose-escalation and dose-expansion cohorts are shown in Table 3 and Table 4, respectively.
Figure 6. Response rates in the dose-escalation cohorts of the phase I/II CC-92480-MM-001 trial*
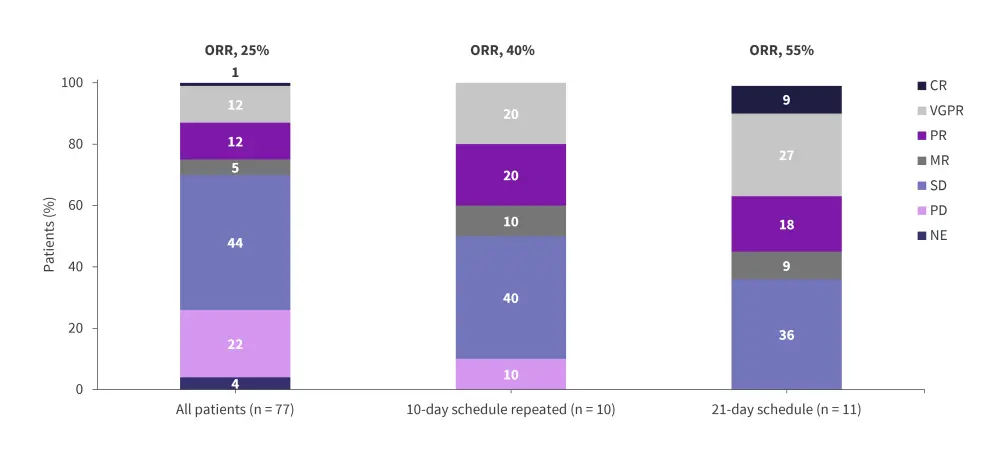
CR, complete response; MR, minimal response; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
*Adapted from Richardson, et al.6
Figure 7. Response rates in the dose-expansion cohorts of the phase I/II CC-92480-MM-001 trial*
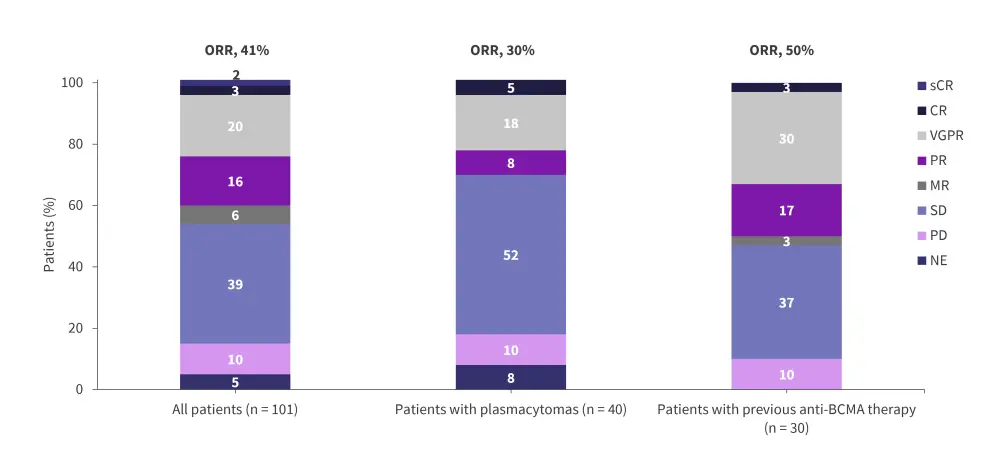
BCMA, B-cell maturation antigen; CR, complete response; MR, minimal response; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
*Adapted from Richardson, et al.6
Table 3. AEs occurring in ≥20% of patients and AEs of interest in the dose-escalation cohort of the phase I/II CC-92480-MM-001 trial*
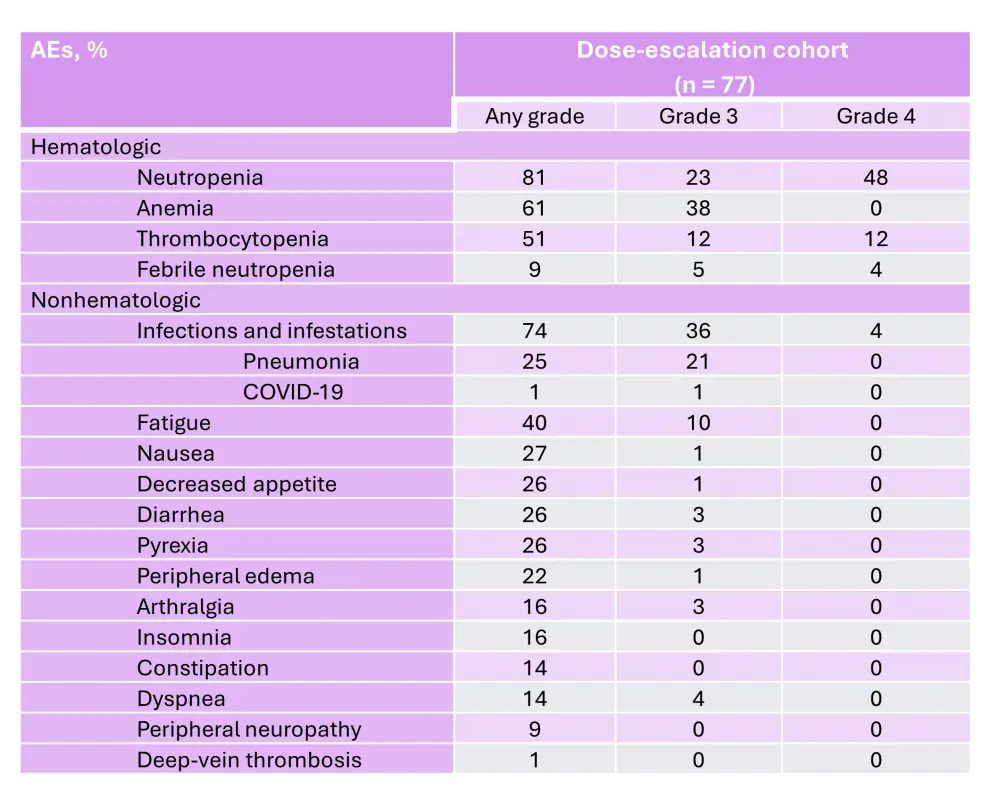
AE, adverse event.
*Adapted from Richardson, et al.6
Table 4. AEs occurring in ≥20% of patients and AEs of interest in the dose-expansion cohort of the phase I/II CC-92480-MM-001 trial*
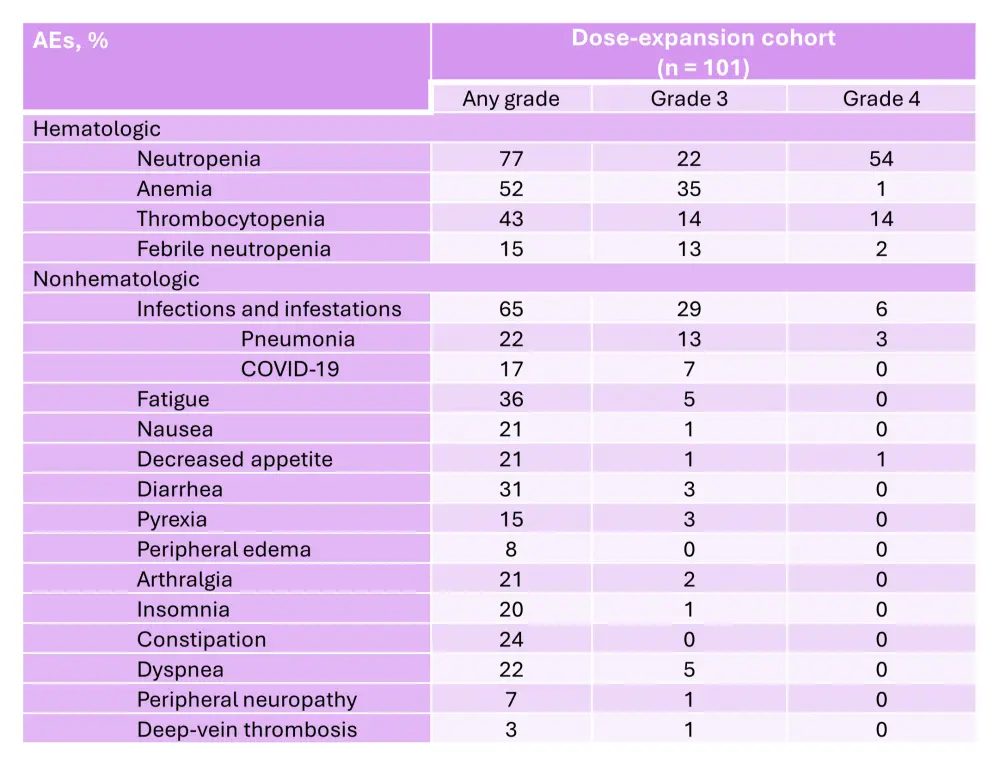
AE, adverse event.
*Adapted from Richardson, et al.6
The ongoing phase I/II CC-92480-MM-002 trial (NCT03989414) is assessing the safety and efficacy of mezigdomide in combination with dexamethasone plus bortezomib (MeziVd), carfilzomib (MeziKd), daratumumab (MeziDd), or elotuzumab (MeziEd) in patients with RRMM.1 Patients in this trial are generally less heavily pretreated than in the CC-92480-MM-001 trial, with a median 1–3 prior lines of therapy.1 Preliminary efficacy data indicate ORRs between 45.0–90.9% across the cohorts (Figure 8).1 The median DoRs were 10.9 months and not reached in the dose-escalation and dose-expansion MeziVd cohorts, respectively, and 12.3 months in the dose-escalation MeziKd cohort. Additionally, among patients who were refractory to prior pomalidomide therapy, the ORR was 76.9% and 83.3% in the MeziVd and MeziKd cohorts, respectively.1 In patients who were refractory to prior lenalidomide and anti-CD38 antibody therapy, the ORR was 69.2%, 75.0%, and 82.4% in the MeziVd dose-escalation, MeziVd dose-expansion, and the MeziKd cohorts, respectively.1 The preliminary safety profiles were consistent with mezigdomide plus dexamethasone, with neutropenia and infections being the most common Grade 3–4 AEs (Figure 9).1
Figure 8. Preliminary response rates in the phase I/II CC-92480-MM-002 trial*
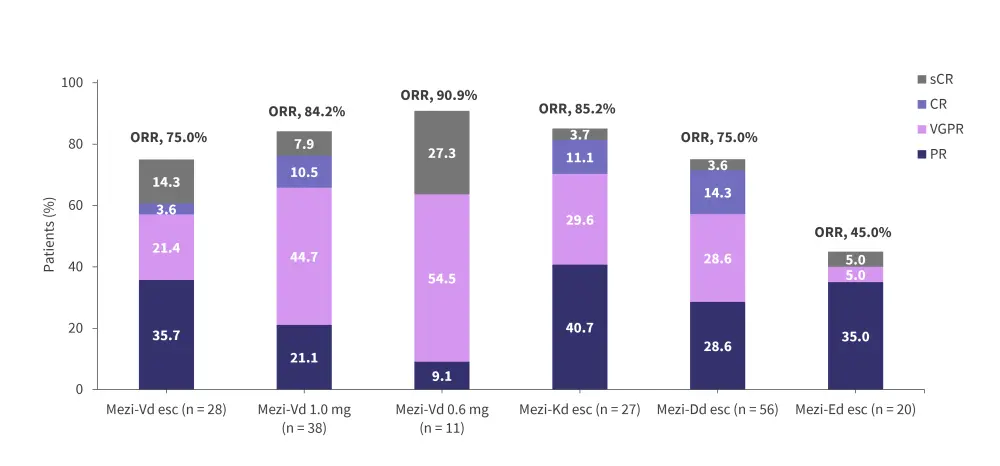
CR, complete response; Dd, daratumumab plus dexamethasone; Ed, elotuzumab plus dexamethasone; esc, dose-escalation cohort; Kd, carfilzomib plus dexamethasone; Mezi, mezigdomide; ORR, overall response rate; PR, partial response; sCR, stringent complete response; Vd, bortezomib plus dexamethasone; VGPR, very good partial response.
*Adapted from Hartley-Brown, et al.1
Figure 9. Incidences of Grade 3–4 neutropenia and infections in the phase I/II CC-92480-MM-002 trial*
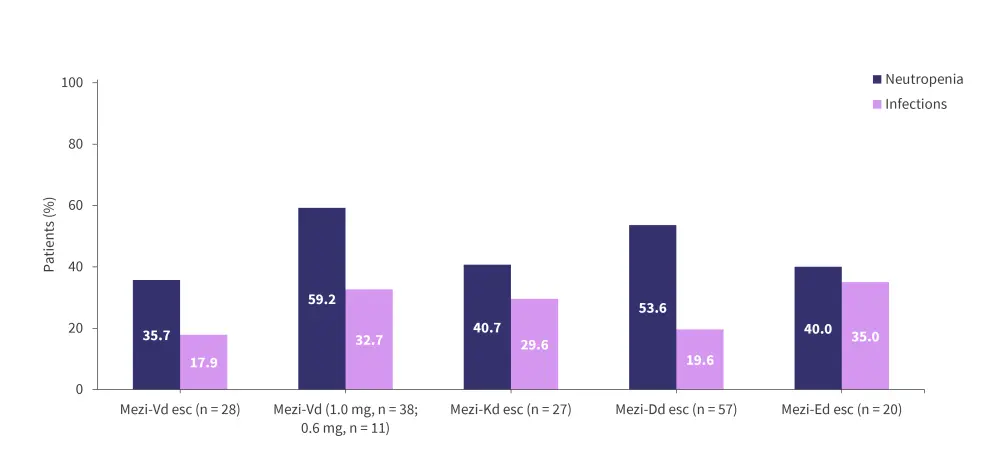
Dd, daratumumab plus dexamethasone; Ed, elotuzumab plus dexamethasone; esc, dose-escalation cohort; Kd, carfilzomib plus dexamethasone; Mezi, mezigdomide; Vd, bortezomib plus dexamethasone.
*Adapted from Hartley-Brown, et al.1
During the Multiple Myeloma Hub Steering Committee Meeting in November 2023, Paul Richardson chaired a discussion on the application of CELMoDs in multiple myeloma.
Patient case discussion on treating with CELMoDs
Phase III trials
Several phase III trials are currently evaluating the safety and efficacy of CELMoDs in patients with RRMM (Figure 10).9–11 Additionally, the EXCALIBUR-Maintenance trial will assess iberdomide in patients with NDMM (Figure 11).12
Figure 10. Phase III studies of CELMoDs in RRMM*
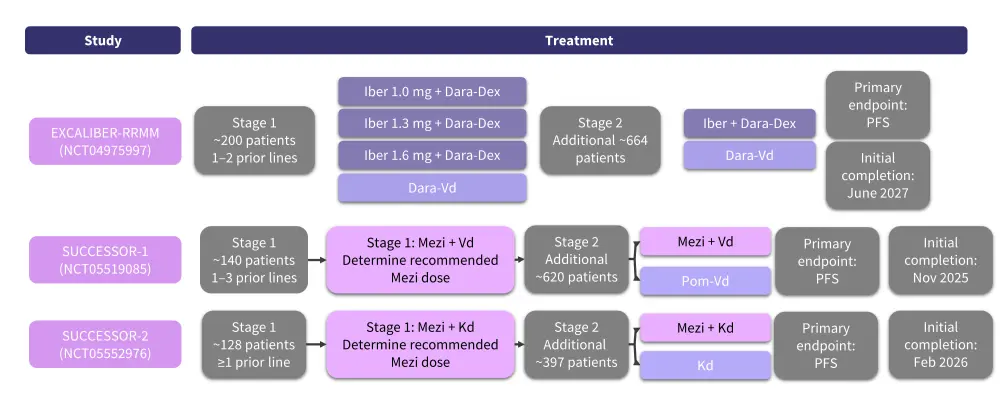
CELMoD, cereblon E3 ligase modulatory drug; Dara, daratumumab; Dex, dexamethasone; Kd, carfilzomib and dexamethasone; iber, iberdomide; Mezi, mezigdomide; PFS, progression-free survival; Pom, pomalidomide; RRMM, relapsed/refractory multiple myeloma; Vd, bortezomib and dexamethasone.
*Adapted from Lonial S, et al.2 and Richardson PG, et al.10,11
Figure 11. Phase III EXCALIBUR-Maintenance trial design*
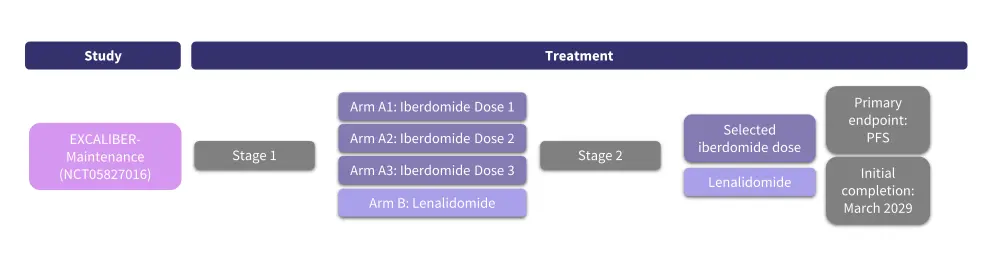
PFS, progression-free survival.
*Adapted from https://clinicaltrials.gov/study/NCT05827016.12
EXCALIBER-RRMM9
The phase III EXCALIBER-RRMM trial (NCT04975997) will assess the efficacy and safety of IberDd vs daratumumab plus bortezomib and dexamethasone (DVd) in patients aged ≥18 years with RRMM and 1–2 prior lines of therapy with a partial response or better to ≥1 prior line of therapy and progressive disease during or after the last regimen. This trial will be conducted in two stages. In Stage 1, ≥200 patients will be randomized 1:1:1:1 to receive iberdomide at 1.0, 1.3, or 1.6 mg plus daratumumab and dexamethasone or DVd to identify the optimal dose of iberdomide in the IberDd regimen. In Stage 2, approximately 664 additional patients will be randomized 1:1 to IberDd at the selected dose or DVd for the safety and efficacy analysis. Patients will be stratified by prior treatment lines (1 vs 2), age (≤70 vs >70 years), and International Staging System (ISS) stage (I–II vs III).
Patients in the IberDd arm will receive 28-day cycles of iberdomide on Days 1–21, plus daratumumab 1,800 mg subcutaneously (SC) on Days 1, 8, 15, and 22 of Cycles 1–2, Days 1 and 15 of Cycle 3–6, and Day 1 of Cycle ≥7, plus dexamethasone 40 mg or 20 mg orally for patients aged ≤75 years and >75 years, respectively, on Days 1, 8, 15, and 22. Patients in the DVd arm will receive 21-day cycles for Cycles 1–8 and 28-day cycles for Cycles ≥9 of daratumumab 1,800 mg SC on Days 1, 8, and 15 for Cycles 1–3 and Day 1 for Cycles ≥4, plus bortezomib 1.3 mg/m2 SC on Days 1, 4, 8, and 11 for Cycles 1–8, and dexamethasone 20 mg or 10 mg orally for patients aged ≤75 years and >75 years, respectively on Days 1, 2, 4, 5, 8, 9, 11, and 12 for Cycles 1–8. Treatment will continue until progressive disease or unacceptable toxicity.
The primary efficacy endpoint is PFS, while secondary endpoints include OS, DoR, time to progression, ORR, measurable residual disease (MRD) negativity rate, safety, and quality of life. Patient enrollment began in June 2022 and is currently ongoing.
SUCCESSOR-110
The phase III SUCCESSOR-1 (NCT05519085) trial will compare the safety and efficacy of mezigdomide vs pomalidomide in patients aged ≥18 years with RRMM and 1–3 prior lines of therapy with a minimal response or better to ≥1 prior line of therapy, no prior pomalidomide exposure, no proteasome inhibitor refractoriness, and progressive disease during or after the last regimen. This trial will be conducted in two stages. In Stage 1, 140 patients will be randomized 1:1:1:1 to receive either mezigdomide 1.0, 0.6, or 0.3 mg, plus dexamethasone and bortezomib, or pomalidomide plus dexamethasone and bortezomib (PVd) to determine the optimal dose of mezigdomide. In Stage 2, an additional 620 patients will be randomized 1:1 to MeziVd or PVd for the safety and efficacy analysis. Patients will be stratified by prior treatment lines (1 vs >1), age (≤70 vs >70 years), and ISS stage (I vs II vs III).
In the MeziVd arm, patients will receive 21-day cycles of mezigdomide on Days 1–14, bortezomib 1.3 mg/m2 SC on Days 1, 4, 8, and 11 for Cycles 1–8 and Day 2 and 8 of Cycle ≥9, and dexamethasone 20 mg orally on Days 1, 2, 4, 5, 8, 9, 11, and 12 for Cycles 1–8 and on Days 1, 2, 8, and 9 of Cycle ≥9. In the PVd arm, patients will receive 21-day cycles of pomalidomide 4 mg orally on Days 1-14 plus Vd as administered in the MeziVd arm. Treatment will continue until progressive disease or unacceptable toxicity.
The primary efficacy endpoint is PFS. Secondary endpoints include the recommended dose of mezigdomide plus Vd, OS, DoR, time to progression, ORR, safety, and quality of life. Patient enrollment began in September 2022 and is currently ongoing.
SUCCESSOR-211
The phase III SUCCESSOR-2 trial (NCT05552976) will evaluate the safety and efficacy of MeziKd vs carfilzomib plus dexamethasone (Kd) in patients aged ≥18 years with RRMM and ≥1 prior line of therapy, including lenalidomide and anti-CD38 antibody therapy, with a minimal response or better to ≥1 prior line of therapy, progressive disease during or after the last regimen, and no prior carfilzomib treatment. This trial will be conducted in two stages. In Stage 1, ≥128 patients will be randomized 3:3:3:2 to mezigdomide 1.0, 0.6, or 0.3 mg plus Kd, or Kd to determine the optimal dose of mezigdomide in combination with Kd. In Stage 2, roughly 397 additional patients will be randomized 3:2 to MeziKd at the selected dose or Kd for the efficacy and safety analysis. Patients will be stratified by prior treatment lines (≤2 vs >2), age (≤70 vs >70 years), and ISS stage (I vs II vs III).
In the MeziKd arm, patients will receive 28-day cycles of mezigdomide on Days 1–21, plus carfilzomib 20 mg/m2 intravenously on Day 1 of Cycle 1 followed by 56 mg/m2 on Days 8 and 15 of Cycle 1 and Days 1, 8, and 15 of Cycles 2–12 and on Days 1 and 15 of Cycles ≥13, and dexamethasone 40 mg orally or intravenously (with 20 mg optional for some patient groups) on Days 1, 8, 15, and 22. In the Kd arm, patients will receive 28-day cycles of carfilzomib 20 mg/m2 intravenously on Days 1 and 2 of Cycle 1 followed by 56 mg/m2 on Days 8, 9, 15, and 16 of Cycle 1 and on Days 1, 2, 8, 9, 15, and 16 of Cycles ≥2, plus dexamethasone 20 mg orally or intravenously (with 10 mg optional for some patient groups) on Days 1, 2, 8, 9, 15, 16, 22, and 23. Treatment will continue until progressive disease or unacceptable toxicity.
The primary efficacy endpoint is PFS. Secondary endpoints include the recommended dose of mezigdomide plus Kd, OS, DoR, time to progression, ORR, safety, and quality of life. Patient enrollment began in October 2022 and is currently ongoing.
EXCALIBUR-Maintenance12
In addition to the phase III trials of CELMoDs in patients with RRMM mentioned above, the phase III EXCALIBUR-Maintenance trial (NCT05827016) will assess the safety and efficacy of iberdomide maintenance therapy vs lenalidomide maintenance therapy following auto-HSCT in patients with NDMM. This trial will include an estimated 1,216 patients with NDMM who received 3–6 cycles of induction therapy including a proteasome inhibitor and immunomodulatory agents with or without anti-CD38 antibody therapy followed by auto-HSCT. Patients must have achieved at least a partial response after auto-HSCT within 12 months of initiating induction therapy with or without consolidation therapy. This trial will assess three doses of iberdomide vs lenalidomide. The primary endpoint is PFS. Secondary endpoints include safety, MRD negativity, OS, time to progression, and quality of life.
Question 1 / 1
What are the primary efficacy endpoints of the phase III EXCALIBUR-RRMM, SUCCESSOR-1, and SUCCESSOR-2 trials?
A
Overall response rate
B
Quality of life
C
Overall survival
D
Progression-free survival
Conclusion
CELMoD therapies have demonstrated promising clinical activity and a tolerable safety profile for the treatment of patients with RRMM.1 Based on the clinical data available, CELMoDs could be an effective treatment option for patients who are refractory to immunomodulatory agents, proteasome inhibitors, and monoclonal antibodies.1 In particular, mezigdomide has demonstrated promising efficacy in patients with clinical features associated with a poor prognosis, including triple-class-refractory disease, high-risk cytogenetics, the presence of extramedullary disease, and prior anti-BCMA therapy.1 While results from the phase II trials of mezigdomide and iberdomide suggest that mezigdomide may be more potent, iberdomide may have a more tolerable safety profile, with lower rates of hematological AEs.5–7 Iberdomide also has the potential to improve post-auto-HSCT outcomes in patients with NDMM as a maintenance therapy.8 Results from the phase III studies discussed above will be instrumental in defining the future applications of CELMoD-based therapies in MM.1 Finally, CELMoDs may prove to be a viable combination partner for both existing and novel therapies, including bispecific antibodies and chimeric antigen receptor T-cell therapies.1
Your opinion matters
As a result of this content, I commit to reviewing clinical data of CELMoDs to guide my treatment of multiple myeloma in clinical practice.
This educational resource is independently supported by BMS. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Paul Richardson
Paul Richardson Sagar Lonial
Sagar Lonial