All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
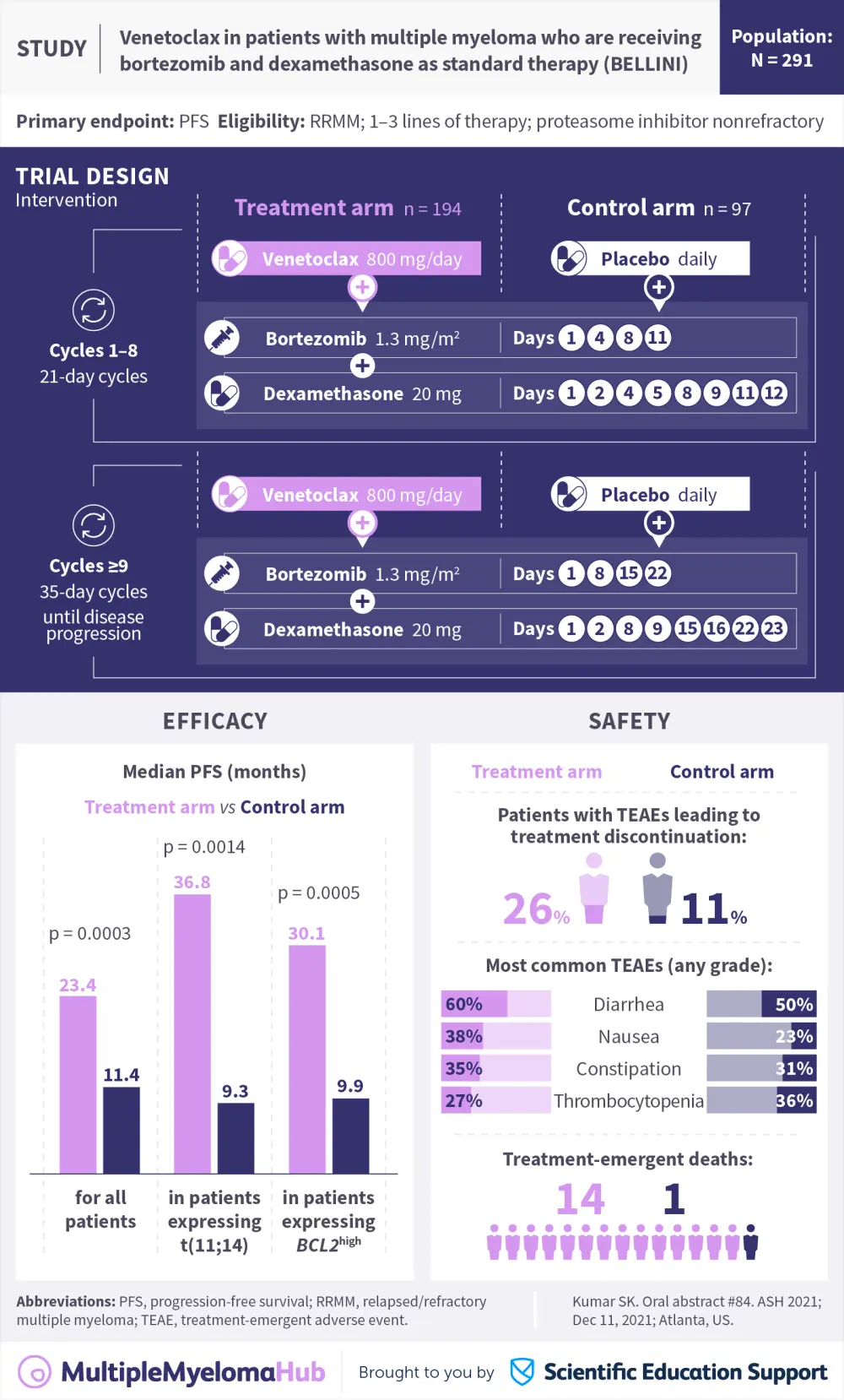
Visual abstract | Venetoclax in patients with multiple myeloma who are receiving bortezomib and dexamethasone as standard therapy (BELLINI)
Multiple myeloma (MM) cells are typified by high levels of antiapoptotic proteins, such as BCL-2.1 Use of BCL-2 inhibitors, such as venetoclax, induces apoptosis. When combined with bortezomib and dexamethasone, the BELLINI phase III study demonstrated that venetoclax improved response rates and progression-free survival (PFS) in patients with relapsed/refractory MM (RRMM), though increased mortality was observed in the venetoclax group (baseline characteristics are detailed here). At the 63rd American Society of Hematology (ASH) Meeting and Exposition, Kumar¹ presented updated safety and efficacy data from the final prespecified overall survival (OS) analysis of the BELLINI trial. This is summarized in the visual abstract presented.
The addition of venetoclax to bortezomib and dexamethasone demonstrated improved PFS after 45.6 months median follow-up.¹ This was more marked in patients with t(11;14) or BCL2high expression and standard-risk cytogenetics. Although the combination resulted in increased early mortality compared with the control arm, the OS hazard ratio improved over time. Interestingly, patients with t(11;14) or BCL2high expression did not seem to be at increased risk of early mortality. These data support a biomarker-driven approach to venetoclax treatment in RRMM.¹
Details of other ongoing trials with venetoclax in MM can be found here.
Visual abstract
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

